Introduction
Recently, there are increasing concerns of energy and environmentalism due, in part, to the growing consumption of non-renewable fossil fuels and the rising atmospheric levels of carbon dioxide (CO2), a major greenhouse gas that is considered to be a major contributor to global warming and other unforeseen severe consequences. The photocatalytic reduction of CO2 into hydrocarbons using solar energy, often referred to as artificial photosynthesis, is believed to be a potential and promising approach to solve both issues.[ Reference Li, Feng, Yan and Zou 1 – Reference Li, An, Park, Khraisheh and Tang 5 ] Since the reduction of CO2 by a TiO2 photocatalyst was reported by Inoue and Fujishima's group,[ Reference Inoue, Fujishima, Konishi and Honda 6 ] the photocatalytic reduction of CO2 has attracted the attention of many researchers. The key to this process is to develop an efficient photocatalyst that can properly position the valence and conduction bands for the oxidation and reduction half-reactions of water and CO2, respectively.[ Reference Li, An, Park, Khraisheh and Tang 5 , Reference Habisreutinger, Schmidt-Mende and Stolarczyk 7 ] To date, various photocatalysts (such as TiO2,[ Reference Liu, Zhao, Andino and Li 8 ] CdS,[ Reference Wang and Wang 9 ] GaP,[ Reference Barton, Rampulla and Bocarsly 10 ] Zn2GeO4,[ Reference Yan, Wang and Zou 11 ] etc.) have been used for photocatalytic CO2 reduction. Usually, the photocatalysts with an ideal perovskite structure are expected to exhibit good photocatalytic activity, which has been demonstrated in many studies.[ Reference Kato and Kudo 12 , Reference Kudo, Kato and Nakagawa 13 ] Recently, a series of perovskite type materials of the form ABO3 (such as BaZrO3,[ Reference Chen, Wang, Huang, Zhang, Zhang, Li and Zou 14 ] BaCeO3,[ Reference Wang, Huang, Chen, Zhang, Li and Zou 15 ] SrTiO3,[ Reference Xie, Umezawa, Zhang, Reunchan, Zhang and Ye 16 ] etc.) have been studied in the photocatalytic reduction of CO2.
Although the photocatalytic reduction of CO2 has been well studied, the conversion efficiency of CO2 is still very low compared with that of water splitting.[ Reference Li, Wen, Low, Fang and Yu 3 , Reference Tu, Zhou and Zou 4 ] Two major photocatalytic systems are being studied: a solid–liquid interface prepared by an aqueous dispersion of photocatalysts and a solid–gas interface.[ Reference Li, An, Park, Khraisheh and Tang 5 ] Usually, both the liquid-phase (CH3OH, HCHO, HCOOH) and gas-phase (CH4) products can be detected in a liquid photoreaction system, whereas only gas-phase (CH4, CO) products could be detected in a gas photoreaction system.[ Reference Habisreutinger, Schmidt-Mende and Stolarczyk 7 ] Although the solid–liquid reaction interface increases the accessible area for carriers and reactants, this approach has two drawbacks: the rather low solubility of CO2 and the reduction of water competing with CO2 reduction in the aqueous suspensions. Although the solid–gas reaction interface promotes the separation of products and reactants, the ratio of gaseous CO2 and H2O is easily tunable, which thus inhibits competitive water reduction.[ Reference Mori, Yamashita and Anpo 17 , Reference Anpo and Chiba 18 ] Because the products of the photocatalytic reduction of CO2 are mostly C1 compounds, it is necessary to eliminate the effects of any organic or carbon residues on the whole photocatalytic reaction process because organic molecules (e.g., acetic acid) or carbon residues may undergo the photo-Kolbe reaction, photodegradation, photolysis reactions or reverse disproportionation reactions on the photocatalyst.[ Reference Izumi 19 , Reference Dey 20 ]
This paper begins with a brief description of the basic concepts of the photocatalytic reduction of CO2 and introduces some experimental problems encountered in the gas photoreaction system. In addition, this paper reviews perovskite oxide semiconductor catalysts used in the photocatalytic reduction of CO2. The prospects for future developments of CO2 photoreduction are also presented.
Basic concepts of the photocatalytic reduction of CO2
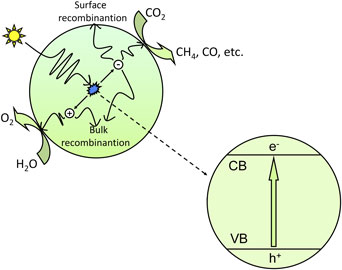
CO2 photoreduction is the conversion of CO2 into hydrocarbon fuels using sunlight, which is also known as artificial photosynthesis. Because CO2 is one of the most thermodynamically stable carbon compounds and because it cannot absorb light in the wavelengths of 200–900 nm, the CO2 photoreduction process requires a suitable photosensitizer, which is generally a semiconductor catalyst.[ Reference Indrakanti, Kubicki and Schobert 21 , Reference Roy, Varghese, Paulose and Grimes 22 ] Figure 1 shows the basic mechanism for the photocatalytic process of CO2 reduction. In the photocatalytic process, photons having energy greater than or equal to the band gap energy are absorbed by the semiconductor, and the electrons in the valence band (VB) are excited to the conduction band (CB). Consequently, equal numbers of electrons and holes are generated in the CB and VB, respectively. The photogenerated electron–hole pairs separate from each other and transfer to the active sites on the surface of semiconductors; then, the photogenerated electrons with strong reduction potential reduce CO2 into hydrocarbons such as CH4 and CO, etc., and the photogenerated holes with strong oxidation potential oxidize H2O into O2.
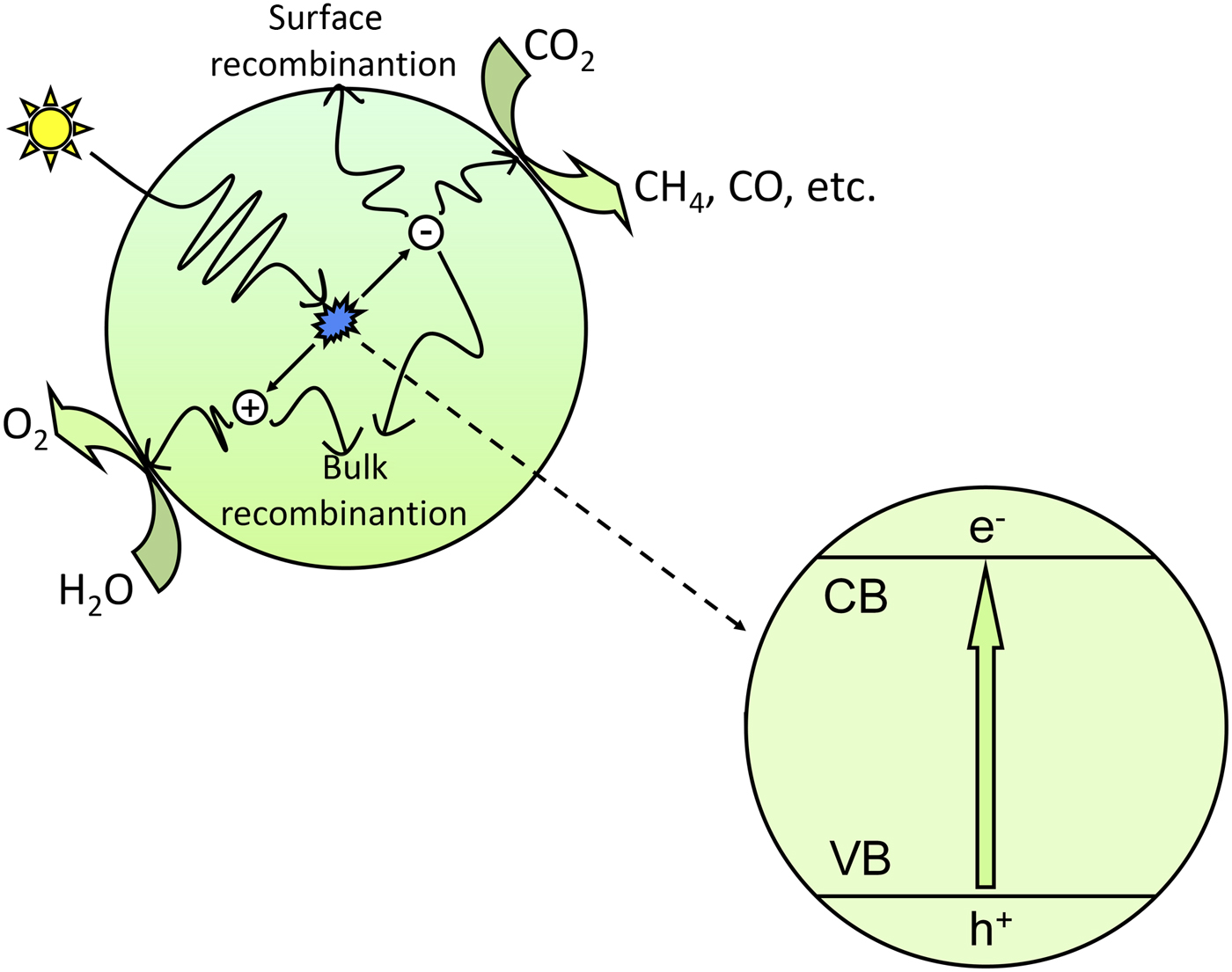
Figure 1. Schematic illustration of the photoexcitation and electron transfer process for the photocatalytic reduction of CO2 with H2O as a reductant.
However, not all photogenerated electron–hole pairs reaching the surface of semiconductor catalysts can participate in the redox reaction, as the energy of the photogenerated electron–hole pairs must meet a certain thermodynamic conditions.[ Reference Li, An, Park, Khraisheh and Tang 5 , Reference Indrakanti, Kubicki and Schobert 21 ] That is, the bottom of the CB of the semiconductor photocatalysts should have a more negative potential than the reduction potential of CO2 forming HCOOH, CO, HCHO, CH3OH and CH4; likewise, the top of the VB should have a more positive potential than the oxidation potential of H2O. Equations (1)–(8) show the possible reactions involved in CO2 photoreduction and their corresponding theoretical potentials (vs. the normal hydrogen electrode in an aqueous solution at pH 7 and 25°C, assuming unit activity).[ Reference Tu, Zhou and Zou 4 , Reference Li, An, Park, Khraisheh and Tang 5 , Reference Indrakanti, Kubicki and Schobert 21 ]
From a thermodynamic point of view,[
Reference Indrakanti, Kubicki and Schobert
21
,
Reference Schneider, Jia, Muckerman and Fujita
23
] the reaction of one electron to form CO2
![]() $^{ \bullet {\rm -}} $
is highly unfavorable due to the very negative reduction potential of −1.9 V (vs. NHE) for this process [Eq. (1)]. Thus, many researchers believe that the photocatalytic reduction of CO2 is a multi-electronic process, which requires less energy for electron transfer compared with a mono-electronic process [Eqs. (2)–(6)]. In the multi-electron CO2 reduction pathway, the reaction requires the assistance of a corresponding number of protons [Eq. (8)]. The formation of products could be different, which is determined by the number of photogenerated electron–hole pairs participating in the redox reaction.
$^{ \bullet {\rm -}} $
is highly unfavorable due to the very negative reduction potential of −1.9 V (vs. NHE) for this process [Eq. (1)]. Thus, many researchers believe that the photocatalytic reduction of CO2 is a multi-electronic process, which requires less energy for electron transfer compared with a mono-electronic process [Eqs. (2)–(6)]. In the multi-electron CO2 reduction pathway, the reaction requires the assistance of a corresponding number of protons [Eq. (8)]. The formation of products could be different, which is determined by the number of photogenerated electron–hole pairs participating in the redox reaction.
Although photocatalytic reduction of CO2 has been well studied, the CO2 conversion efficiency of this process is still very low. In addition to a suitable E g and CB potential, many factors influence the conversion efficiency of the multi-electron CO2 photocatalytic reduction process. These factors include excitation, transport, separation (recombination) of the photogenerated electron–hole pairs, the adsorption capacity of the gas molecules, the reduction of CO2, the oxidation of H2O, and so on. Furthermore, the selective formation of specific products is also a considerable problem in the photocatalytic reduction of CO2.[ Reference Li, Wen, Low, Fang and Yu 3 , Reference Tu, Zhou and Zou 4 ] So far, many strategies, including increased visible-light excitation (e.g., impurity doping, sensitization,[ Reference Sato, Morikawa, Saeki, Kajino and Motohiro 24 ] and solid solution construction[ Reference Zhang, Ouyang, Kako and Ye 25 ]), improved transport and separation of the photogenerated electron–hole pairs (e.g., loading with cocatalysts,[ Reference Ishitani, Inoue, Suzuki and Ibusuki 26 ] synthesis of nanostructured photocatalysts,[ Reference Liu, Torimoto, Matsumoto and Yoneyama 27 ] and construction of semiconductor heterojunctions[ Reference Xi, Ouyang and Ye 28 ]), enhanced adsorption of CO2 (e.g., increasing the surface area[ Reference Wang, Huang, Liu, Sun and Li 29 ]), and suppression off-target side reactions (e.g., the competitive reaction of H2O reduction in Eq. (7)[ Reference Zhai, Xie, Fan, Zhang, Wang, Deng and Wang 30 ]), etc., have been designed to achieve a high efficiency and selectivity.
Key points of the experimental details
The efficiency of the photocatalytic reduction of CO2 depends not only on the factors mentioned above, a suitable semiconductor photocatalyst (with proper E g, CB and VB), the excitation, transport and separation (recombination) of the photogenerated electron–hole pairs, etc., but also on the experimental conditions,[ Reference Dey 20 , Reference Yahaya, Gondal and Hameed 31 – Reference Zhang, Han, Hong and Yu 33 ] such as the light source, response time, reaction temperature, the ratio of H2O and CO2, etc.
Photocatalytic reduction of CO2 is mainly carried out at room pressure and temperature and is controlled by a cooling water recirculation system. A high pressure mercury lamp and/or a 300 W xenon lamp, usually with a cutoff filter, are the most common light sources in CO2 photoreduction. Yahaya et al. found that the traditional UV lamp had a drawback in that the light intensity would decrease with rising temperature due to the lengthy illumination time, and this decrease affects the efficiency of CO2 photocatalytic reduction.[ Reference Yahaya, Gondal and Hameed 31 ] The selection of response time for the studies of CO2 photoreduction is different, ranging from tens of minutes to hours. Zhao et al. studied nano-sized CoPc/TiO2 particles as a catalyst for photoconversion of CO2 and discussed the effect of irradiation time on the formation of photo-reduced products over a period of 6–50 h.[ Reference Zhao, Fan, Liu and Wang 32 ] At short reaction times, the product yields increased rapidly because more photoelectrons were generated with increasing time of light exposure; however, the product yields flattened out after 20 h of irradiation. This result can be attributed to saturation of the reactive surface active sites absorbed with intermediate products. In addition, the molar ratio of H2O and CO2 is also found to play an important role in determining the reaction rate and product selectivity for CO2 photocatalytic reduction. Zhang et al. investigated the photocatalytic reduction of CO2 with H2O on a Pt-loaded TiO2 catalyst and studied the effect of the H2O/CO2 molar ratio on CH4 yield. They found that the CH4 yield of 0.15 wt.% Pt-loaded TiO2 nanotube increased as the mole ratio of H2O and CO2 increased, while the different H2O:CO2 molar ratios had little effect on the CH4 yield of 0.12 wt.% Pt-loaded TiO2 nanoparticles.[ Reference Zhang, Han, Hong and Yu 33 ] The formation of CH4 occurred mainly due to the high concentration of the surface OH group, which had been shown previously by Ikeue et al.[ Reference Ikeue, Nozaki, Ogawa and Anpo 34 ]
The main products in a gas phase reaction system for photocatalytic reduction of CO2 are generally CH4, which is usually on the μmol scale, as well as a small amount of CO.[ Reference Liu, Zhao, Andino and Li 8 , Reference Chen, Wang, Huang, Zhang, Zhang, Li and Zou 14 – Reference Xie, Umezawa, Zhang, Reunchan, Zhang and Ye 16 ] As we mentioned above, the photocatalytic reduction of CO2 is a complex multi-electronic reaction process. There are two plausible pathways for CH4 formation[ Reference Tu, Zhou and Zou 4 , Reference Dey 20 ]:
It is necessary to verify whether the carbon source for CH4 production is actually the CO2 for the gaseous CO2 reduction because any organic adsorbates or carbon residues may take part in side reactions, such as the photo-Kolbe reaction, mineralization reaction, photolysis reaction or reverse disproportionation reaction.[ Reference Izumi 19 , Reference Dey 20 ] These reactions could create a false impression of the formation of hydrocarbon products.
If there are any carbon residues deposited or buried on the surface of catalyst during the preparation of the catalyst for the photocatalytic reduction of CO2, a reverse disproportionation reaction of the impurity might occur in the presence of CO2 in the reaction system. In this case, the generated CO would react with the water adsorbed on the surface active sites of the catalyst; then, the generated CH3OH would ultimately generate CH4. This pathway has been shown by Yang et al. through the combined use of in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and 13C labeled CO2 during CO2 photoreduction over Cu-promoted crystalline TiO2 catalysts. The reaction process could be described as follows[ Reference Izumi 19 , Reference Yang, Yu, van der Linden and Wu 35 ]:
Yui et al. demonstrated that organic adsorbates, especially CH3COOH, had a significant effect on the formation of CH4 in CO2 photoreduction over TiO2. The organic adsorbed on the surface of TiO2 could participate in a so-called photo-Kolbe reaction (CH3COOH, for example)[ Reference Yui, Kan, Saitoh, Koike, Ibusuki and Ishitani 36 ]:
In addition, if there are any alcohols and O2 in the CO2 photoreduction reaction system producing CH4, CO2 would be generated in-situ due to the mineralization of alcohols on the surface of the semiconductor photocatalyst, which could be explained through the following mechanism[ Reference Dey 20 ]:
In conclusion, evaluations of the products for CO2 photocatalytic reduction should be interpreted with caution. In other words, the C source should be carefully investigated with respect to the production of CH4 and CO from CO2 in a gaseous CO2 photoreduction system. Most researchers carry out a series of contrast experiments with different conditions including H2O, CO2, irradiation and catalyst to exclude the effects of carbon residues and organic adsorbates on the formation of hydrocarbon products[ Reference Chen, Wang, Huang, Zhang, Zhang, Li and Zou 14 – Reference Xie, Umezawa, Zhang, Reunchan, Zhang and Ye 16 ]; some other studies, however, reported a more precise and intuitive method: isotopic labeling (13C and 18O).[ Reference Yang, Yu, van der Linden and Wu 35 – Reference Li, Zhou, Li, Xu, Meng, Wang, Xiao and Zou 37 ] This approach can simultaneously evaluate the C source and the O source. To guarantee the reliability of the results of CO2 photocatalytic reduction, carbon residues or organic adsorbates must be avoided throughout the entire reaction system. These residues and adsorbates may form during the synthesis of the catalyst if it involves the use of alkoxides and organic solvents (e.g., polyethylene glycol, PEG) as precursors or in washing steps if organic cleaning solvents are used.[ Reference Yang, Yu, van der Linden and Wu 35 , Reference Kočí, Obalová, Matějová, Placháa, Lacnýa, Jirkovskýc and Šolcová 38 , Reference Lin and Frei 39 ] These residues could be removed using particular methods, such as thermal treatment in a continuous flow of N2 or Ar inert gas or at high temperature in air, as well as prolonged washing in a water environment.[ Reference Izumi 19 , Reference Yang, Yu, van der Linden and Wu 35 , Reference Yui, Kan, Saitoh, Koike, Ibusuki and Ishitani 36 ]
Perovskite oxide semiconductor photocatalysts for gaseous CO2 reduction
An ideal semiconductor photocatalyst for CO2 photoreduction should benefit from: (1) a suitable E g such that the semiconductor absorbs incident photons over a range of wavelengths to generate electron–hole pairs; (2) effective separation between the photogenerated electron–hole pairs that then transfer to the surface of semiconductors and subsequently take part in the oxidation-reduction reaction with CO2 and H2O absorbed on the surface active sites of the semiconductors; and (3) a well-tuned CB and VB such that the bottom of the CB is located at a more negative potential than the reduction potentials of CO2 and such that the top of the VB has a more positive potential than the oxidation potential of H2O. Based on these principles, various photocatalysts, including metal oxides (e.g., TiO2,[ Reference Liu, Zhao, Andino and Li 8 ] ZnO,[ Reference Mahmodi, Sharifnia, Rahimpour and Hosseini 40 ] BiVO4,[ Reference Liu, Huang, Dai, Zhang, Qin, Jiang and Whangbo 41 ] and Ga2O3 [ Reference Park, Choi, Choi, Lee and Kang 42 ]), metal sulfides (e.g., CdS[ Reference Wang and Wang 9 ] and ZnS[ Reference Fujiwara, Hosokawa, Murakoshi, Wada and Yanagida 43 ]), metal phosphides (e.g., GaP[ Reference Barton, Rampulla and Bocarsly 10 ]), nitrides (e.g., C3N4 [ Reference Mao, Peng, Zhang, Li, Ye and Zan 44 ]), etc., have been reported for CO2 reduction. Among them, perovskite oxide semiconductor photocatalysts are good candidates for CO2 reduction due to their unique physical and chemical properties, including their stability, their controllable structure and their ability to be doped in two different cation sites. In addition, perovskite structures are expected to exhibit good photocatalytic activity as has been demonstrated in many studies.[ Reference Kato and Kudo 12 , Reference Kudo, Kato and Nakagawa 13 ] Recently, a series of the perovskite type materials of the form ABO3 (such as BaZrO3,[ Reference Chen, Wang, Huang, Zhang, Zhang, Li and Zou 14 ] BaCeO3,[ Reference Wang, Huang, Chen, Zhang, Li and Zou 15 ] SrTiO3,[ Reference Xie, Umezawa, Zhang, Reunchan, Zhang and Ye 16 , Reference Zhou, Guo, Li, Fan, Zhang and Ye 45 , Reference Kou, Gao, Li, Yu, Zhou and Zou 46 ] etc.) have been studied for the photocatalytic reduction of CO2. Table I shows the summary of perovskite oxide semiconductors used in the photocatalytic reduction of CO2 in a gas reaction system. These semiconductors were roughly divided into five types: tantalates, niobates, titanates, zirconates and cerates.
Tantalates
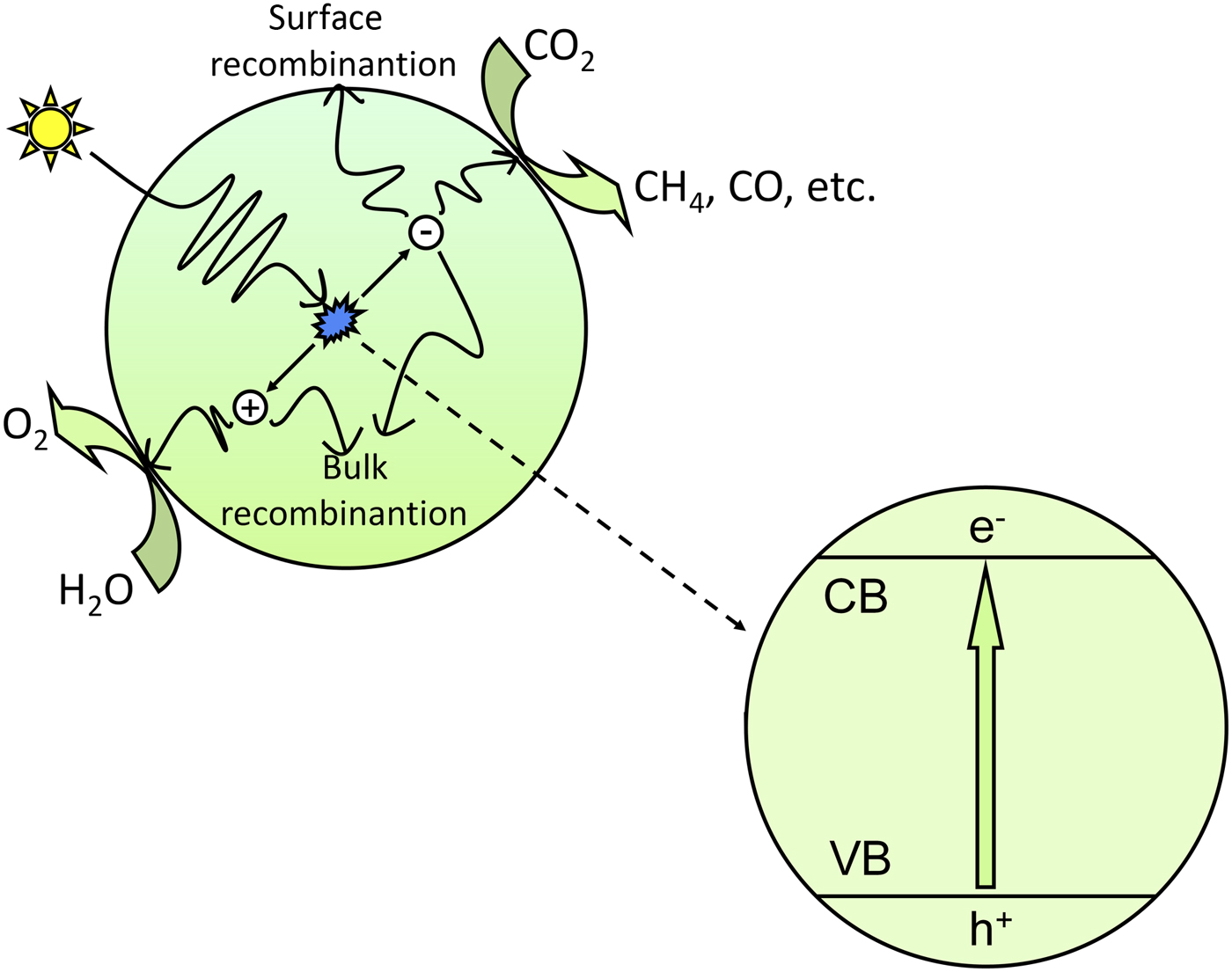
Teramura et al. carried out the photoreduction of CO2 over ATaO3 (A = Li, Na and K) synthesized by a conventional solid-state reaction in the presence of H2. CO was the only gaseous product over all of the samples tested under 200 W Hg-Xe lamp irradiation.[ Reference Teramura, Okuoka, Tsuneoka, Shishido and Tanaka 54 ] LiTaO3 presented the highest activity for CO yield (0.42 µmol/g in 24 h), and the trend of the photocatalytic activities were consistent with that of the E g (LiTaO3 > NaTaO3 > KTaO3). In addition, they also found that the amount of evolved CO gas depended on the amount of chemisorbed CO2 (as shown in Fig. 2),[ Reference Teramura, Okuoka, Tsuneoka, Shishido and Tanaka 54 ] which meant that the adsorption of CO2 had an effect on the reduction efficiency of CO2. H2 was used as reductant in this photocatalytic reduction of CO2 process, whereas other gas reaction systems usually used H2O as reductant.
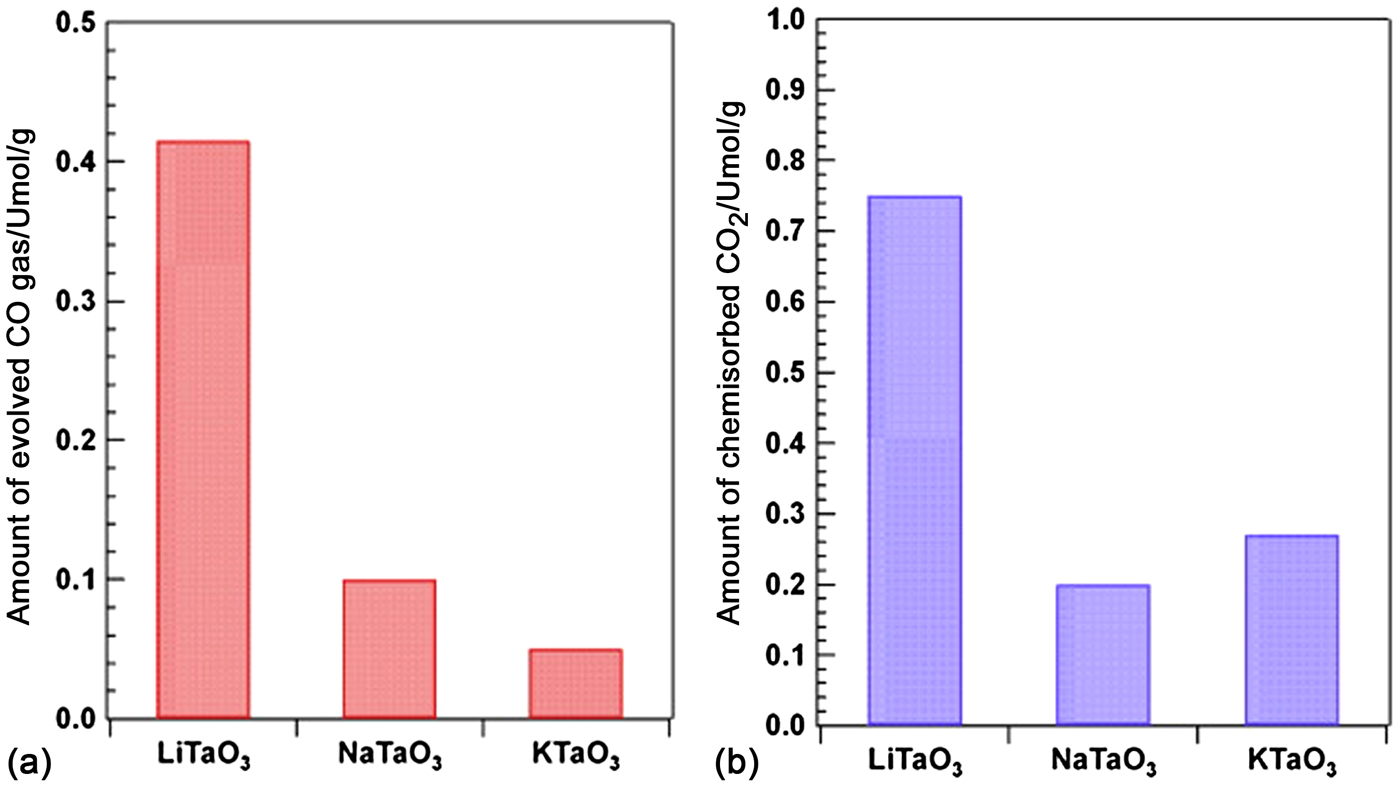
Figure 2. (a) Amount of evolved CO gas for the photocatalytic reduction of CO2 in the presence of H2 as a reductant over ATaO3 (A = Li, Na, K) after 24 h of photoirradiation. (b) Amount of Chemisorbed CO2 on ATaO3 (A = Li, Na, K). (Copyright 2010, Applied Catalysis B: Environmental.[ Reference Teramura, Okuoka, Tsuneoka, Shishido and Tanaka 54 ]).
Niobates
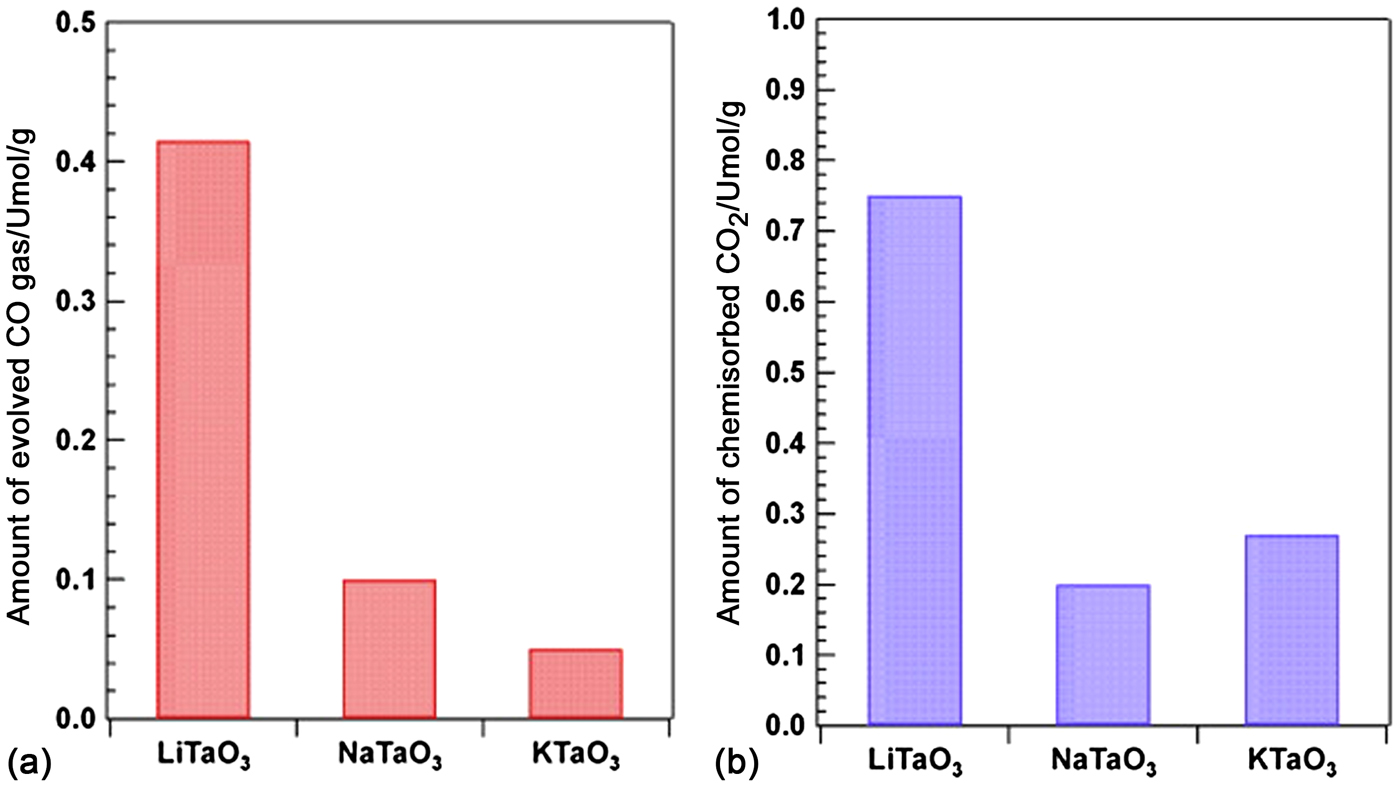
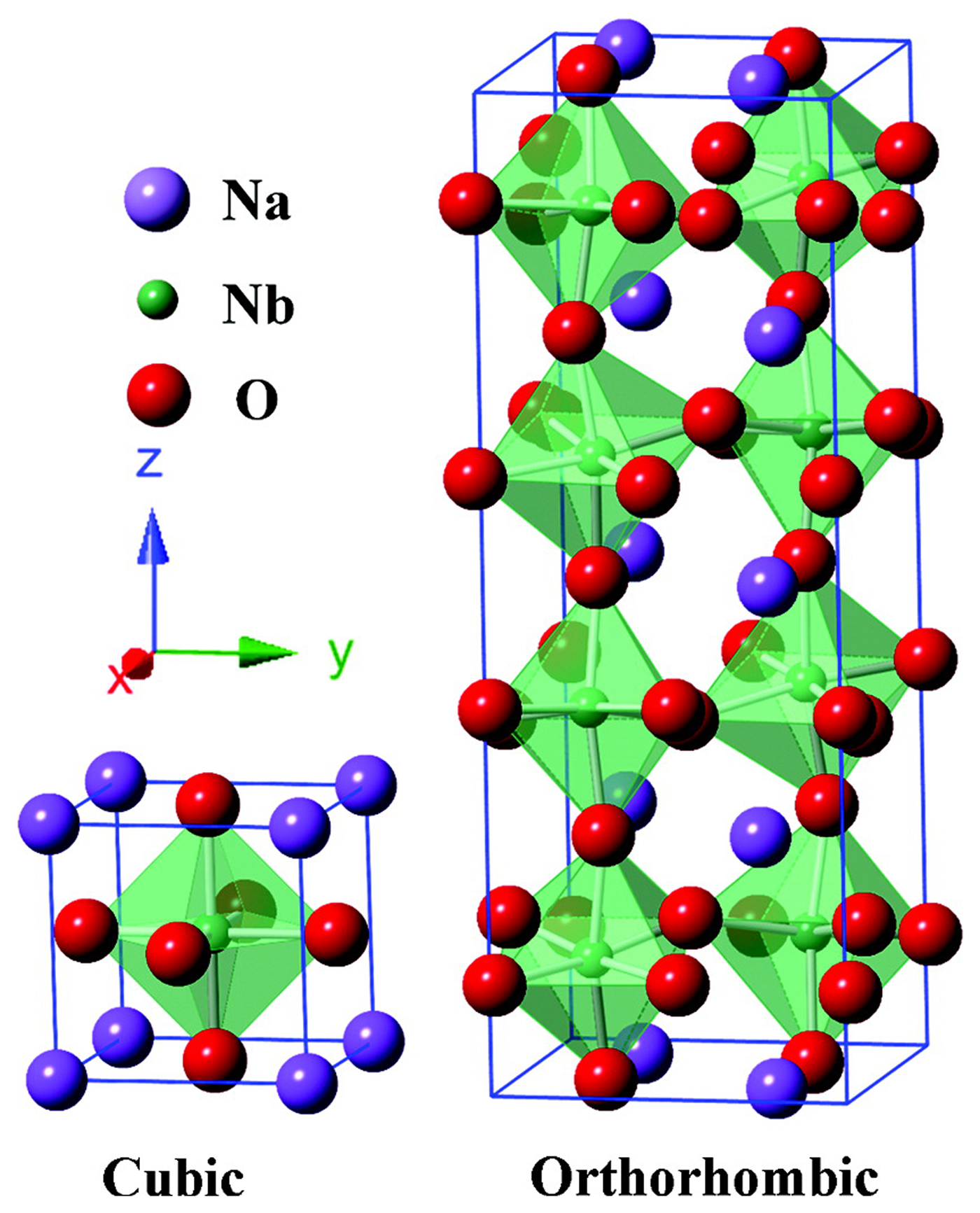
Niobates with a perovskite structure share many characteristics with tantalates and have been used in CO2 photocatalytic reduction systems with water as the reductant. Shi et al. studied the photocatalytic reduction activities of CO2 into CH4 over NaNbO3 samples upon irradiation from a Xe arc lamp. It was discovered that the CH4 evolution rate (653 ppm/h/g) of Pt-loaded NaNbO3 nanowires prepared by a hydrothermal method were much higher than that of bulk NaNbO3 particles synthesized by a solid-state reaction.[ Reference Shi, Wang, Chen, Zhu, Ye and Zou 53 ] Subsequently, they prepared alkali niobate ANbO3 (A = K, Na) photocatalysts using a conventional solid-state reaction method and compared their photocatalytic performance for CO2 reduction under similar conditions. They found that KNbO3 (7.0 ppm/h) showed a higher photocatalytic activity than NaNbO3 (2.3 ppm/h).[ Reference Shi and Zou 52 ] The perovskite NaNbO3 studied in most research has a typical cubic structure, whereas NaNbO3 also has another crystal structure—an orthorhombic structure. The schematic crystal structures of cubic and orthorhombic NaNbO3 are shown in Fig. 3.[ Reference Li, Ouyang, Xi, Kako and Ye 51 ] Li et al. discussed the photocatalytic activities of CO2 reduction over the cubic and orthorhombic NaNbO3 in a gas phase system and found that the CO2 reduction activity over the cubic NaNbO3 was nearly twice that of the orthorhombic NaNbO3. These results might be due to the unique electron structure of cubic NaNbO3, which may support electron excitation and transfer.[ Reference Li, Ouyang, Xi, Kako and Ye 51 ] In addition, they studied the influence of the preparation temperature on the activity of NaNbO3 photocatalyst.[ Reference Li, Ouyang, Zhang, Kako and Ye 50 ] The cubic NaNbO3 prepared at 500°C presented the best photocatalytic performances, and the CH4 evolution for gas phase CO2 photoreduction over NaNbO3 loaded with 0.5 wt.% Pt under a 300 W Xe lamp (λ > 300 nm) could reach 12.6 µmol/m2/h.
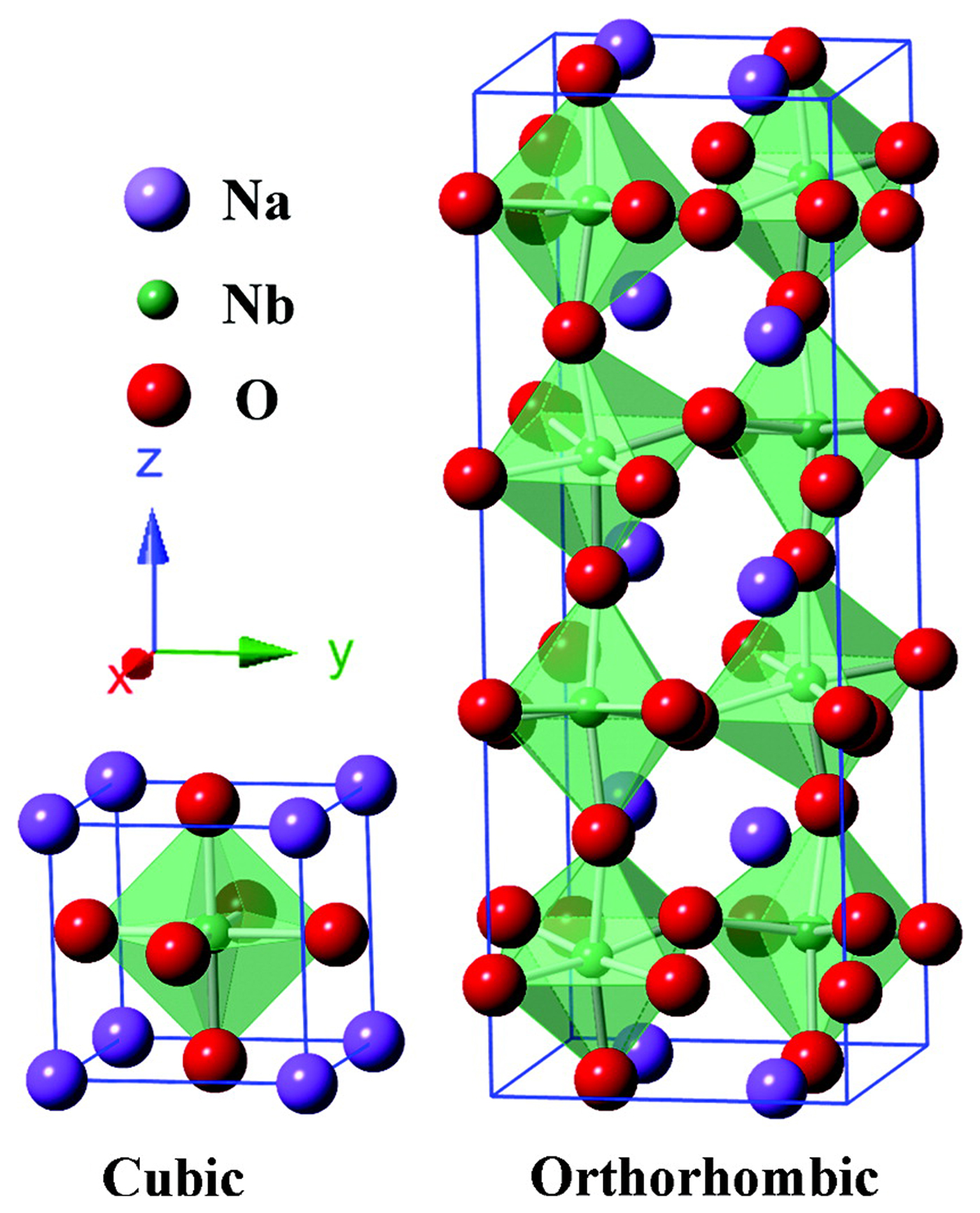
Figure 3. Schematic crystal structures of cubic and orthorhombic NaNbO3. (Copyright 2012, The Journal of Physical Chemistry.[ Reference Li, Ouyang, Xi, Kako and Ye 51 ]).
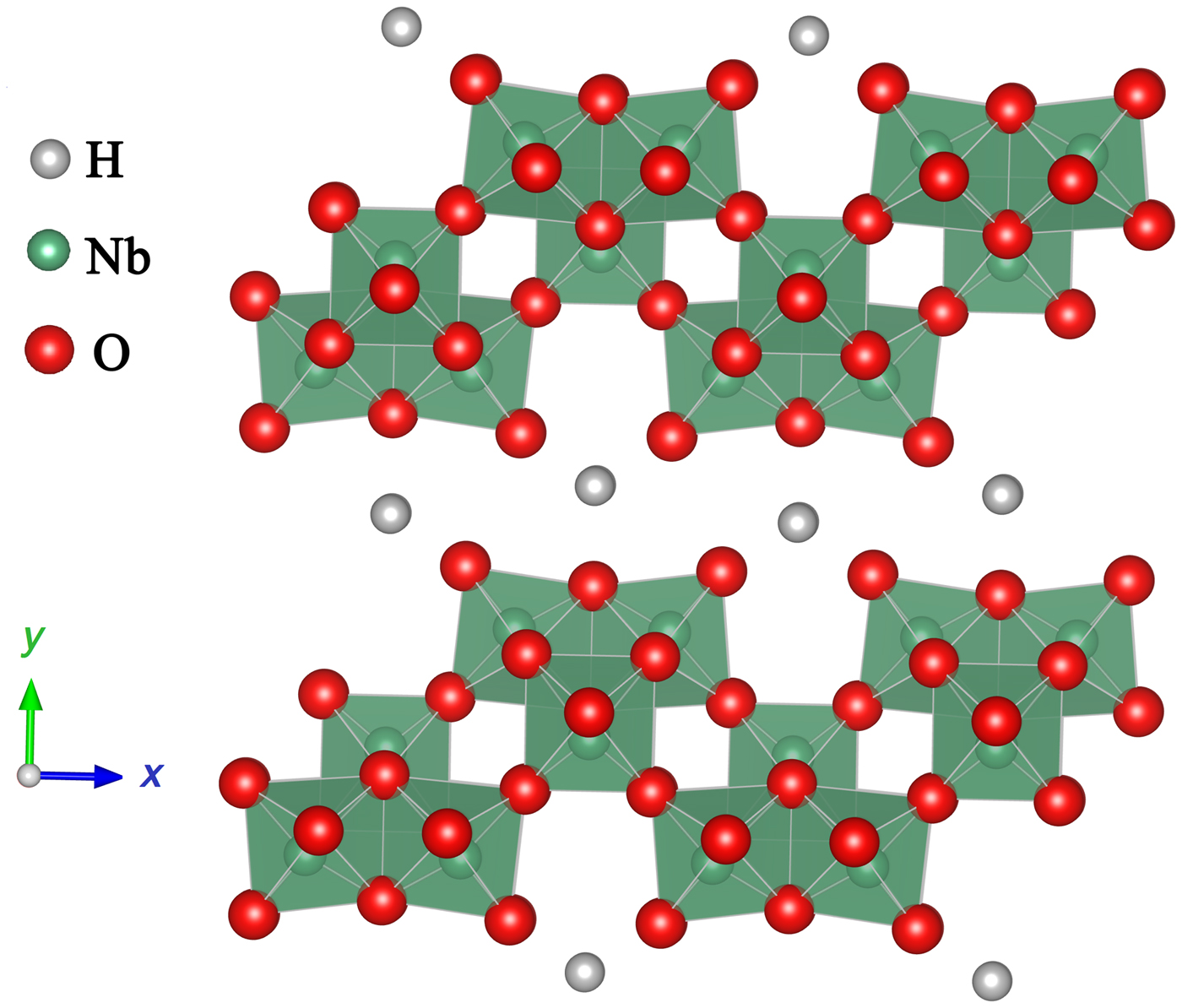
Moreover, differing from the simple ABO3-type perovskite semiconduction photocatalysts, lamellar perovskite niobates, such as HNb3O8 and KNb3O8, have also been applied in the gas phase photocatalytic reduction of CO2.[ Reference Li, Pan, Li and Zhuang 48 , Reference Li, Li, Zhuang, Zhong, Li and Wang 49 ] Figure 4 shows a schematic drawing of the layered structures of HNb3O8 composed of 2D Nb3O8 − anion thin sheets of edge- and corner-shared NbO6 octahedral with H+ intercalated between the layers. Li et al. found that hydrothermally synthesized HNb3O8 and KNb3O8 nanobelts exhibited much higher yields of CH4 compared with the HNb3O8 and KNb3O8 particles prepared by conventional solid-state reactions as well as commercial TiO2.[ Reference Li, Pan, Li and Zhuang 48 ]
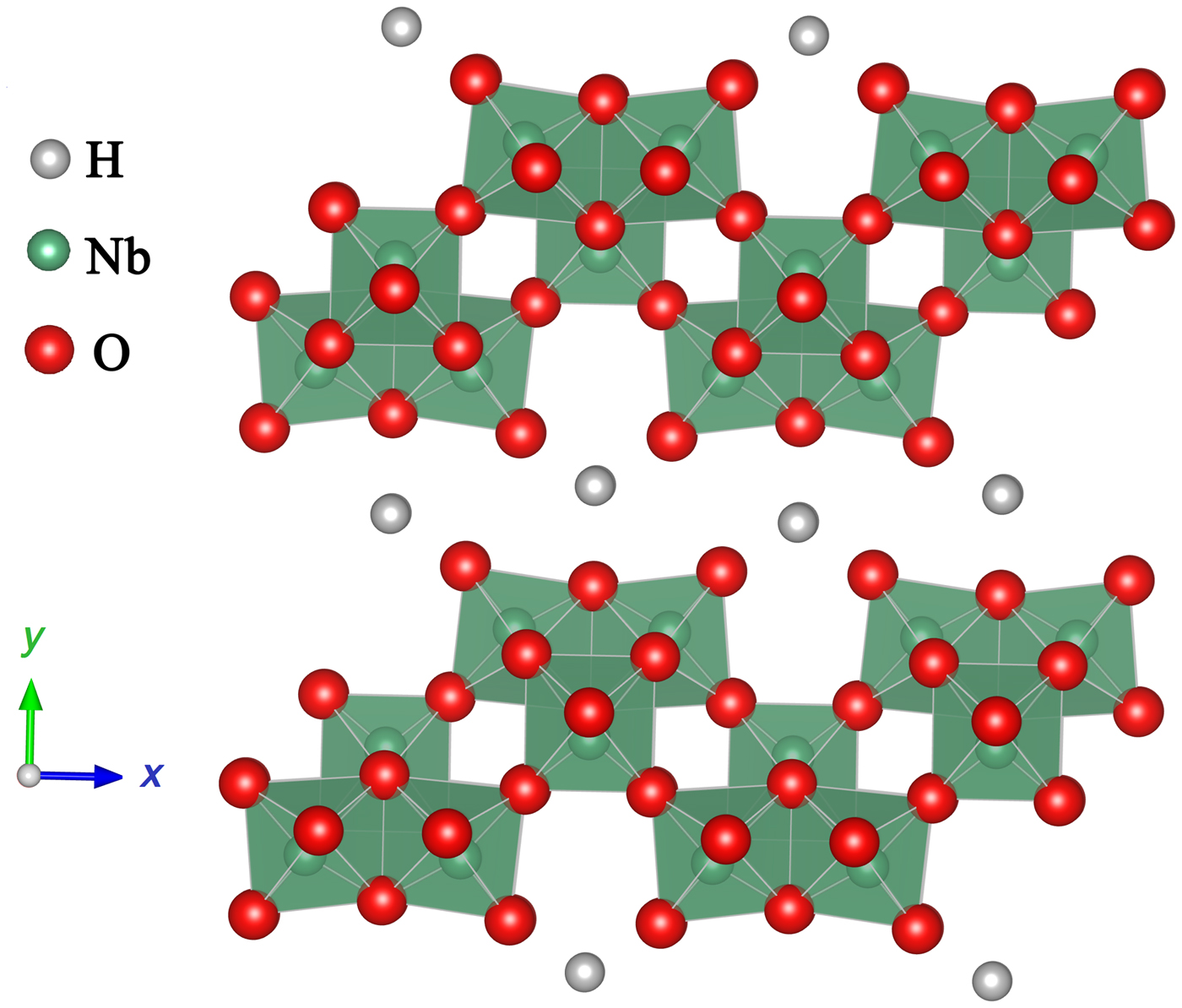
Figure 4. Schematic drawing of the layered structures of HNb3O8.
Titanates
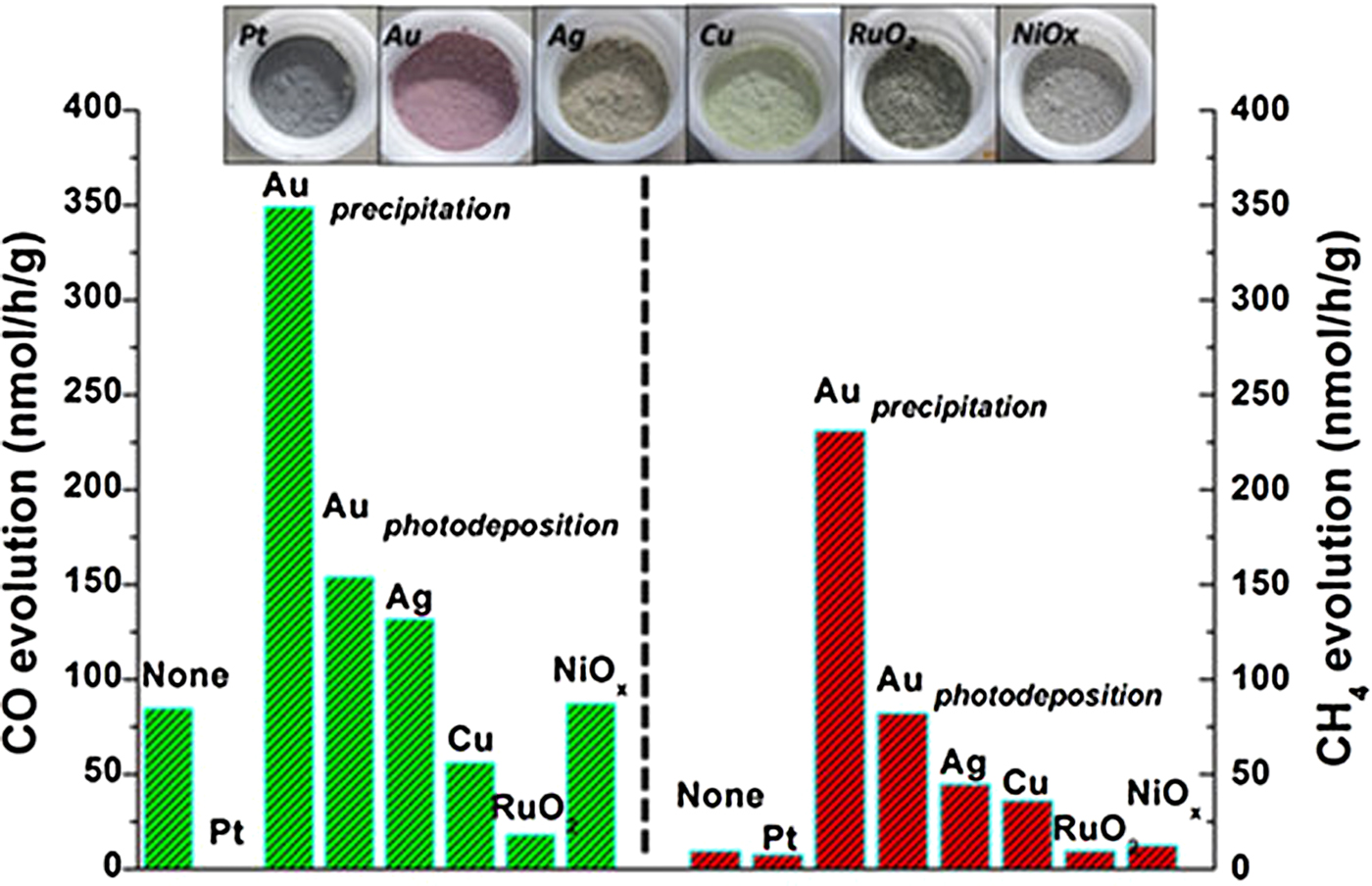
Several perovskite titanates, such as SrTiO3, PbTiO3 and CaTiO3, have also been explored for gas phase CO2 photocatalytic reduction.[ Reference Xie, Umezawa, Zhang, Reunchan, Zhang and Ye 16 , Reference Zhou, Guo, Li, Fan, Zhang and Ye 45 – Reference Kwak and Kang 47 ] Among them, SrTiO3 is the most widely studied photocatalysts. Xie et al. prepared a self-doped SrTiO3−δ using a carbon-free one-step combustion, and they demonstrated that the chemical adsorption of CO2 could be improved by increasing the oxygen deficiency of the SrTiO3. This deficiency promoted the photocatalytic activity of CO2 reduction to generate CH4 under visible light irradiation.[ Reference Xie, Umezawa, Zhang, Reunchan, Zhang and Ye 16 ] Zhou et al. discussed a leaf-shaped 3D hierarchical artificial photosynthetic system of ATiO3 (A = Sr, Ca and Pb) prepared by a modified sol-gel method using fresh green leaves as a structure template. They studied the effects of various cocatalysts (Au, Ag, Cu, Pt, RuO2 and NiO x ) on the photocatalytic activity of CO2. As shown in Fig. 5, CH4 and CO were the two main products, and Au as a suitable cocatalyst exhibited the best performance for the selectivity of both CH4 and CO (using SrTiO3 as an example).[ Reference Zhou, Guo, Li, Fan, Zhang and Ye 45 ] In another study, Kou et al. synthesized a highly active photocatalyst with response to visible light by substituting the Ti4+ in SrTiO3 to transition metal (Co, Fe, Ni) ions for gas phase CO2 reduction, and Pt-SrTi0.98Co0.02O3 had the highest activity (the yield of CH4 was 63.6 ppm/h).[ Reference Kou, Gao, Li, Yu, Zhou and Zou 46 ]
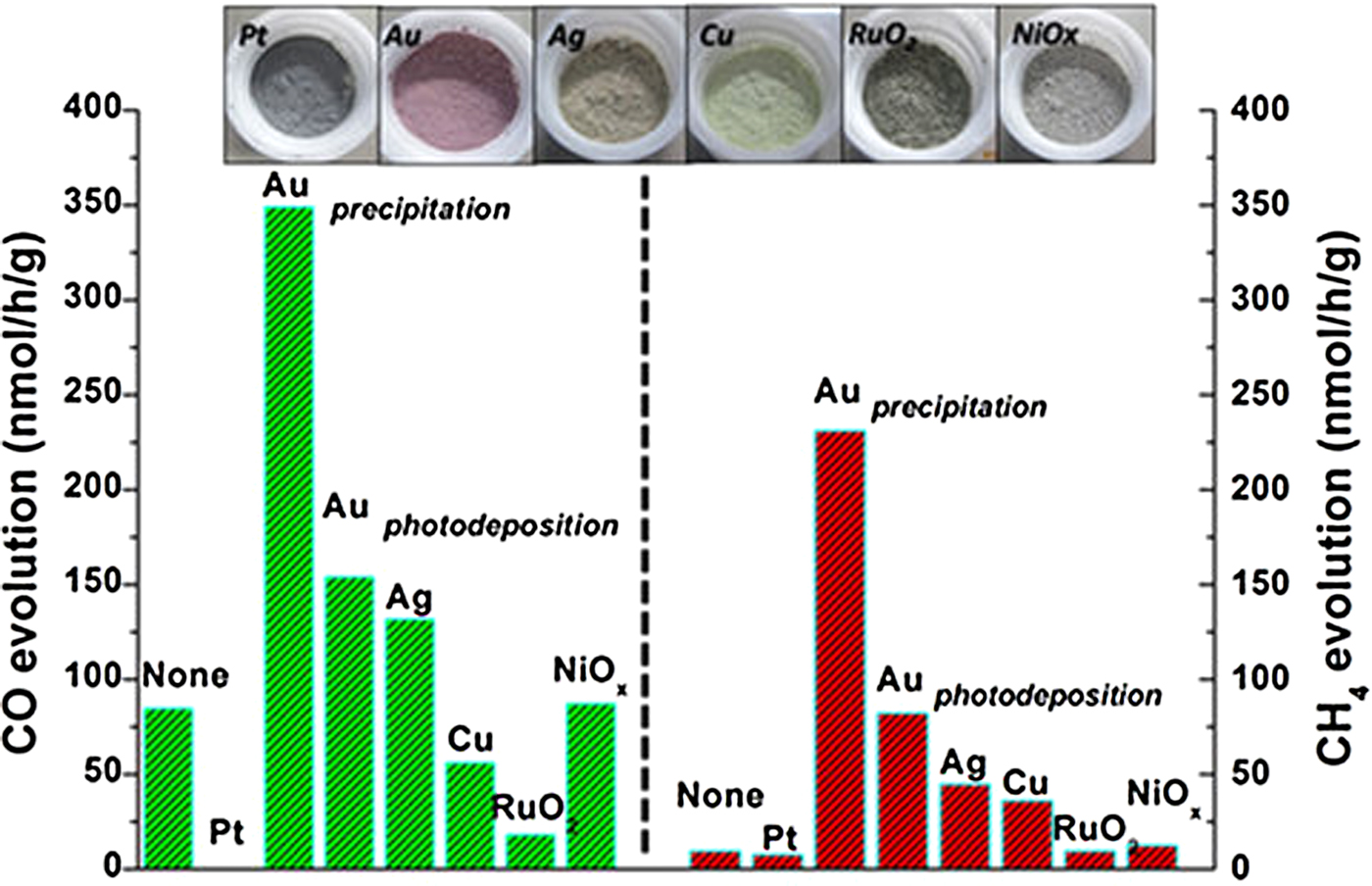
Figure 5. CO and CH4 evolution on an artificial photosynthetic system of SrTiO3 of CO2 photoreduction loaded with different cocatalysts. (Copyright 2013, Scientific Reports.[ Reference Zhou, Guo, Li, Fan, Zhang and Ye 45 ]).
Zirconates
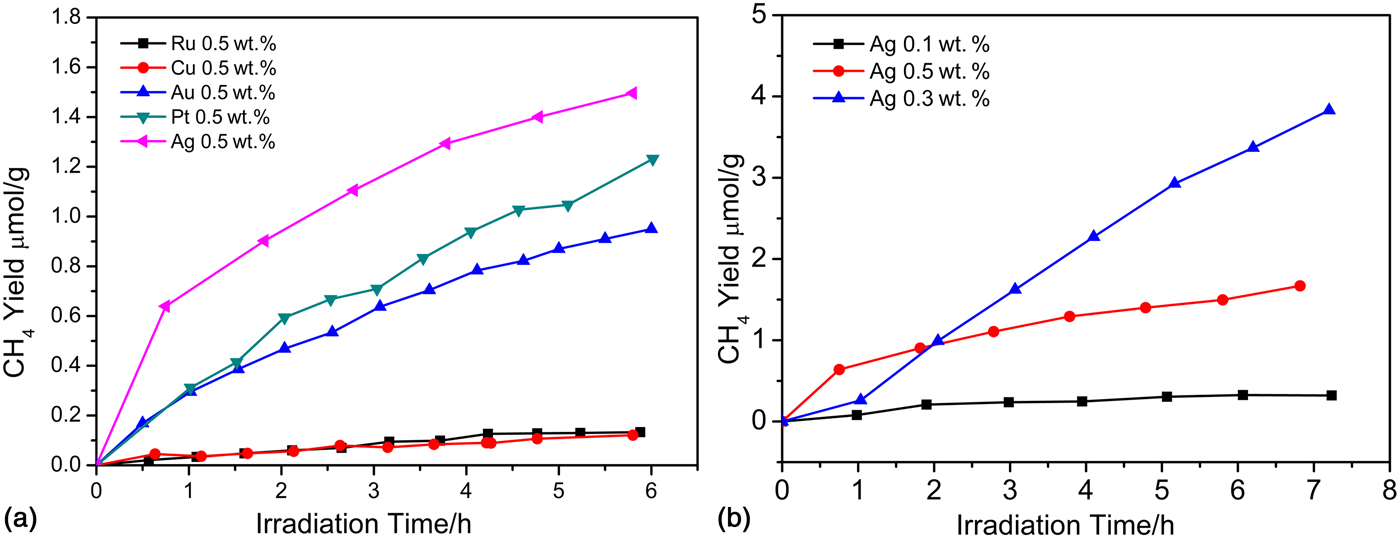
Apart from the above-mentioned tantalates, niobates and titanates photocatalysts, Chen et al. first investigated the photocatalytic properties of BaZrO3 for CO2 reduction into CH4 in a gas reaction system.[ Reference Chen, Wang, Huang, Zhang, Zhang, Li and Zou 14 ] Barium zirconate made by the Pechini process has a bandgap of 4.8 eV, which means that BaZrO3 only has a UV light response. They also confirmed the effects of different cocatalysts on the photocatalytic activities and optimized the loaded amount of metal nanoparticle cocatalysts. As shown in Fig. 6, Ag was a suitable cocatalyst that exhibited the best selectivity for producing CH4 with the BaZrO3 system. In addition, a CH4 yield of up to 0.57 µmol/h/g was obtained when 0.3 wt.% Ag cocatalyst was loaded onto the surface of the BaZrO3. In this case, the Ag site acted as an electron trap to effectively decrease the recombination rate of the photogenerated electron–hole pairs.
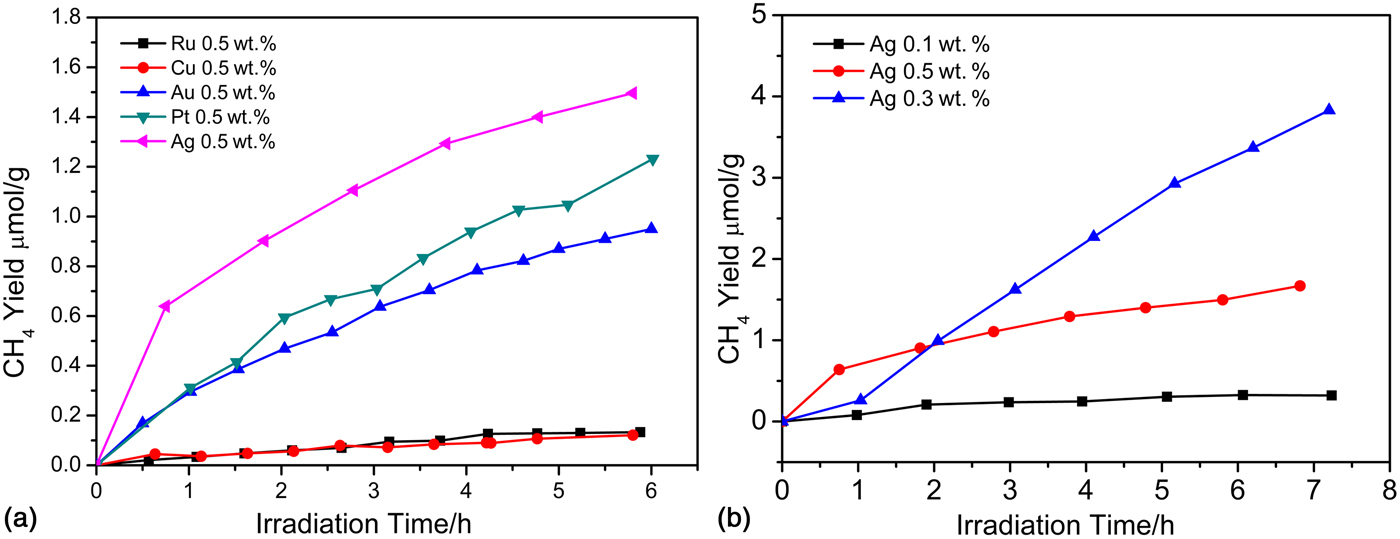
Figure 6. (a) CH4 yields of BaZrO3 deposited with different cocatalysts (Ru, Cu, Au, Pt, Ag) of the same apparent amount. (b) CH4 yields of BaZrO3 with different amounts of Ag deposition. (Copyright 2015, Catalysis Science & Techonology.[ Reference Chen, Wang, Huang, Zhang, Zhang, Li and Zou 14 ]).
Cerates
All the perovskite oxide semiconductor photocatalysts for CO2 reduction mentioned above consist of metal cations with d0 (Zr4+, Ti4+, Nb5+, or Ta5+) configurations. Wang et al. first reported the photocatalytic properties of BaCeO3, whose conduction band mainly consists of Ce 4f orbitals, as a new photocatalyst for gas phase CO2 reduction.[ Reference Wang, Huang, Chen, Zhang, Li and Zou 15 ] The activity of photocatalytic reduction of CO2 into CH4 over BaCeO3 loaded with 0.3 wt% Ag cocatalyst was up to 0.55 µmol/h/g. This experiment proved that perovskite-type oxide semiconductor photocatalysts with conduction bands dominated by 4f electronic configuration could also react as well as the semiconductor photocatalysts with d0 electronic configuration in gas phase CO2 photoreduction.
Summary and outlook
The photocatalytic reduction of CO2 into renewable hydrocarbon fuels using solar energy is a promising approach for simultaneously achieving carbon cycling and solving the shortage of sustainable energy. An ideal semiconductor photocatalyst for CO2 photoreduction should have a suitable E g and proper positioning of the CB and VB, which should satisfy thermodynamic conditions of CO2 reduction. In addition, the photogenerated electron–hole pairs should separate effectively and transfer to the surface active sites of the semiconductor photocatalysts so that they can participate in the oxidation-reduction reactions with CO2 and H2O.
The photocatalytic reduction of CO2 is a complex multi-electronic reaction process. The main products in a gas phase CO2 reduction are generally CH4 as well as a small amount of CO, and the yield of CH4 is usually on the μmol scale. This yield is relatively low compared with the conversion of water splitting.[ Reference Chen, Wang, Huang, Zhang, Zhang, Li and Zou 14 – Reference Xie, Umezawa, Zhang, Reunchan, Zhang and Ye 16 , Reference Zhou, Guo, Li, Fan, Zhang and Ye 45 – Reference Teramura, Okuoka, Tsuneoka, Shishido and Tanaka 54 ] The conversion efficiency and products selectivity of gaseous CO2 photoreduction not only depends on the characteristics of the photocatalyts but also depends on some experimental conditions, such as the light source, response time, reaction temperature, the ratio of H2O and CO2, etc. It is necessary to verify whether the formation of hydrocarbon products is actually from the photocatalytic reduction of CO2 or is from some side reactions with organic adsorbates (e.g., acetic acid) or carbon residues. These adsorbates or residues may be formed during the photo-Kolbe reaction, mineralization reaction or reverse disproportionation reaction in photocatalytic reaction. A series of contrast experiments with different conditions (including H2O, CO2, irradiation and catalyst) or a more intuitive and precise method—isotopic labeling (13C and 18O)—is necessary to detect and/or exclude the effects of carbon residues and organic adsorbates. These contaminants can also be removed by thermal treatment in air or inert gas or by prolonged washing with water upon the formation of products.
Based on the research and the current development of related theories and practical applications, the photocatalytic reduction of CO2 still has a long way to go. The future studies on CO2 photoreduction should be initiated in the following areas.
Developing a new suitable photocatalyst
As we mentioned above, a wide variety of perovskite oxide semiconductors system have been applied to the photocatalytic reduction of CO2 in a gas reaction system under UV or visible light irradiation. Most of them consist of metal cations with d0 configurations including tantalates, niobates, titanates, zirconates, with the exception being cerates such as BaCeO3 with 4f orbitals electron configurations. It is believed that perovskite oxide semiconductor photocatalysts still have great prospects due to their unique physical and chemical properties in the future study of the photocatalytic reduction of CO2. For example, alkaline earth stannates of MSnO3 (M = Ca, Sr and Ba), which contain Sn4+ ions with a d10 electronic configuration, have been used as the photocatalyst applied to photocatalytic reactions for H2 and O2 evolution in water splitting.[ Reference Zhang, Tang and Ye 55 ] The bottom of the CBs are located at −1.46, −1.37 and −0.69 V (versus NHE, pH = 7) for CaSnO3, SrSnO3 and BaSnO3, respectively, while the top of the VBs are 2.98, 2.69 and 2.41 V (versus NHE, pH = 7), respectively. Thus, the photogenerated electrons in the CB are more negative than the reduction potentials of CO2 into HCOOH, CO, HCHO, CH3OH and CH4, and the corresponding photogenerated holes in the VB can easily oxidize H2O in the ABO3 structure of these stannates. In other words, MSnO3 (M = Ca, Sr and Ba) is theoretically feasible as a photocatalyst for the reduction of CO2. In addition, our group has already tested the photocatalytic reduction of CO2 over BaSnO3, and we hope that MSnO3 (M = Ca, Sr and Ba) will be a promising photocatalyst for the gas phase photoreduction of CO2 in future.
In addition, double perovskite oxides with the general chemical formula of A2BB'O6 have attracted much interest due to their variable crystal structures, flexible chemical compositions and other unique physicochemical properties such as being semi-metallic. These catalysts have been successfully applied to water splitting, such as Ba2InTaO6 with a E g of ca. 4.17 eV.[ Reference Song, Yang, Jiang, Gao, Cong and Yang 56 ] A variety of elements are available for substitution into the A or B site; B site elements are usually transition metals with d-orbital configurations, and A site elements are usually alkaline earth ions such as Ca, Sr and Ba.[ Reference Li, Gao, Lei, Xie, Deng and Hu 57 ] Based on these results, development of new double perovskite oxide semiconductors system seem to be one feasible direction for research on CO2 reduction in future.
Cocatalysts
It is generally believed that cocatalysts play a crucial role in semiconductor-based photocatalysis.[ Reference Li, Wen, Low, Fang and Yu 3 , Reference Tu, Zhou and Zou 4 , Reference Yang, Wang, Han and Li 58 ] Firstly, loaded cocatalysts could serve as electron traps to promote the separation of photogenerated electron–hole pairs resulting from the formation of a Schottky barrier between the semiconductor and the cocatalyst. Hence, they could improve the photocatalytic activity and selectivity for CO2 reduction. Secondly, cocatalysts could effectively lower the activation barrier of CO2 due to their better conductivity and lower over potential.[ Reference Osterloh and Parkinson 59 , Reference Maeda and Domen 60 ] To date, a series of cocatalysts including Pd, Pt, Au, Ag, Ru, Cu, Ni, Co, etc. have been used for photocatalytic reactions, and each presented different effects on the conversion efficiency.[ Reference Ishitani, Inoue, Suzuki and Ibusuki 26 , Reference Zhou, Guo, Li, Fan, Zhang and Ye 45 , Reference Fukuzumi, Hong and Yamada 61 ] In addition, it is worth noticing that the bimetallic cocatalyst systems (such as Cu and Pt) have also exhibited good activities for CO2 reduction,[ Reference Varghese, Paulose, LaTempa and Grimes 62 ] and the enhancement mechanism is still unclear. Thus, it is essential to better understand the photocatalytic mechanism of cocatalysts and to develop cheap, abundant and highly efficient cocatalyst systems (e.g., non-noble metal cocatalysts and new-type bifunctional cocatalysts with a proper structure) for the photocatalytic reduction of CO2.
Gas adsorption ability on the surface of photocatalysts
It is well known that the reduction of CO2 and the oxidation of H2O are the two main half-reaction steps for the photocatalytic reduction of CO2. On one hand, effective adsorption of CO2 is an important process that can be promoted by increasing surface area,[ Reference Wang, Huang, Liu, Sun and Li 29 ] by introducing basic sites such as MgO, or by introducing surface oxygen vacancies[ Reference Xie, Umezawa, Zhang, Reunchan, Zhang and Ye 16 , Reference Xie, Wang, Zhang, Deng and Wang 63 ]. Optimization of adsorption is important for improving the conversion efficiency for the photocatalytic reduction of CO2. The adsorption of CO2 molecules on stoichiometric and oxygen-deficient SrTiO3 has been theoretically and experimentally studied by Xie et al. They demonstrated that chemical adsorption of CO2 molecules could be improved by increasing the oxygen deficiency in SrTiO3, thus enhancing the photocatalytic performance for CO2 reduction.[ Reference Xie, Umezawa, Zhang, Reunchan, Zhang and Ye 16 ] On the other hand, water oxidation favors the efficient separation of the photogenerated electron–hole pairs, thus improving the photocatalytic activity for CO2 reduction.[ Reference Li, Wen, Low, Fang and Yu 3 , Reference Izumi 19 ] In addition, deactivation of the photocatalysts may occur after long reaction times, which may be attributed to the active sites gradually becoming covered by the adsorption of the reduction intermediate products or the final products accumulation on the photocatalyst surface over time.[ Reference Zhao, Fan, Liu and Wang 32 , Reference Xiong, Zhao, Zhang and Zheng 64 ] In conclusion, it is important to refine the research on the adsorption of gaseous reactants (CO2, H2O) and the products on the surface of photocatalysts.
In short, the future development for the photocatalytic reduction of CO2 remains challenging, and the comprehensive efforts (such as materials synthesis, doping, nanostructuring and heterojunction)[ Reference Tu, Zhou and Zou 4 , Reference Zhang, Ouyang, Kako and Ye 25 ] are needed to achieve a breakthrough in the conversion efficiency of CO2 reduction into hydrocarbon fuels that may eventually lead to industrialization.
Table I. Summary of perovskite oxide semiconductors for the photocatalytic reduction of CO2 in a gas reaction system.
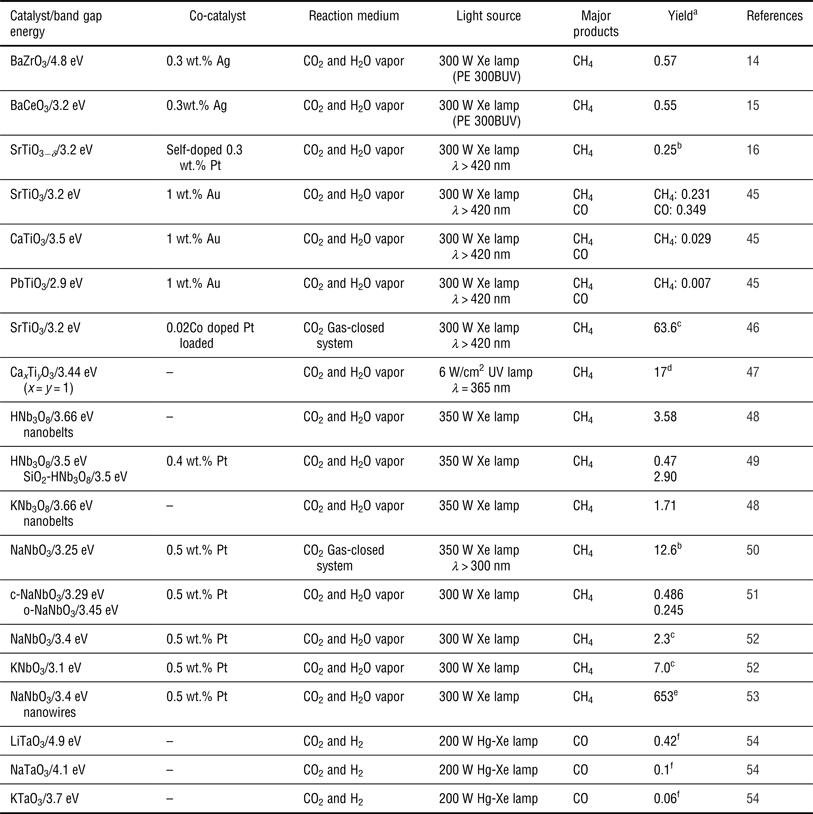
a Maximum formation rate reported for the products in μmol/h/g, unless stated otherwise.
b In μmol/m2 cata/h.
c In ppm/h.
d The entire yield for the main products in μmol/g (7 h, x = y = 1).
e In ppm/h/g.
f The entire yield for the main products in μmol/g (24 h).
Acknowledgments
This work is supported by National Basic Research Program of China (973 Program, 2013CB632404), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, New Century Excellent Talents in University (NCET-12-0268), and the National Natural Science Foundation of China (Nos. 21473090 and 51272102).