The development of highly active cathode materials is essential to lower the operating temperature of solid oxide fuel cells (SOFCs), where the slow kinetics of the oxygen surface exchange on the cathode surface limits the efficiency of SOFCs at intermediate temperatures (500–750 °C).[ Reference Shao and Haile 1 , Reference Steele and Heinzel 2 ] Current cathode materials such as La1−x Sr x MnO3−δ (LSM113)[ Reference da Conceicao, Silva, Ribeiro and Souza 3 – Reference Ioroi, Hara, Uchimoto, Ogumi and Takehara 5 ] with high electronic conductivity but low ionic conductivity[ Reference Adler 6 ] are inadequate for the usage in the intermediate temperature range due to insufficient surface activity.
La1−x Sr x Fe1−y Co y O3−δ (LSCF113), which has beneficial materials properties such as high ionic and electronic conductivity,[ Reference Wang, Katsuki, Dokiya and Hashimoto 7 ] and fast oxygen surface exchange,[ Reference Katsuki, Wang, Dokiya and Hashimoto 8 ] therefore, has been developed as one of the most promising commercial cathode materials for intermediate temperature SOFCs. In particular, a solution infiltration process, in which a phase transition occurs from a liquid into a solid has been widely used to further enhance the surface activity of LSCF113.[ Reference Liu, Ding, Blinn, Li, Nie and Liu 9 – Reference Zhu, Ding, Li, Lu, Su and Zhen 12 ] Utilizing infiltrated LSM113 coatings, it has been shown the enhanced electrocatalytic activity of LSCF113 cathodes.[ Reference Lynch, Yang, Qin, Choi, Liu, Blinn and Liu 11 , Reference Zhu, Ding, Li, Lu, Su and Zhen 12 ] Infiltrated La0.4875Ca0.0125Ce0.5O2−δ (LCC)[ Reference Liu, Ding, Blinn, Li, Nie and Liu 9 ] and Sm0.5Sr0.5CoO3−δ (SSC)[ Reference Lou, Wang, Liu, Yang and Liu 10 ] coatings have also been used for better stability and activity of LSCF113 electrodes. Although many studies have shown the enhanced cathodic performance of LSCF113 by surface modification through a solution-based infiltration process, the origin responsible for the enhanced stability and activity of decorated LSCF113 cathode is poorly understood.
Ruddlesden-Popper (RP) phases (A 2 BO4) have been utilized as a material for the La1−x Sr x CoO3−δ (LSC113) surface modification, which results in the enhanced surface activity of LSC113 significantly due to the formation of heterostructured oxide interfaces.[ Reference Crumlin, Ahn, Lee, Mutoro, Biegalski, Christen and Shao-Horn 13 – Reference Sase, Hermes, Yashiro, Sato, Mizusaki, Kawada, Sakai and Yokokawa 18 ] Using well-defined epitaxial thin film systems, remarkably enhanced oxygen surface exchange kinetics (up to ~2 orders of magnitude) of LSC113 has been reported by decorating (La0.5Sr0.5)2CoO4±δ (LSC214) phase on the LSC113 surface.[ Reference Crumlin, Ahn, Lee, Mutoro, Biegalski, Christen and Shao-Horn 13 , Reference Crumlin, Mutoro, Ahn, la O, Leonard, Borisevich, Biegalski, Christen and Shao-Horn 19 ] Coherent Bragg rod analysis (COBRA) and density functional theory (DFT) have suggested that the enhanced oxygen surface exchange kinetics may be attributed to the Sr segregation at the LSC214–LSC113 interface and the LSC214 surface, resulting from a large driving force for A-site cation interdiffusion across the heterostructured interface.[ Reference Feng, Crumlin, Hong, Lee, Mutoro, Biegalski, Zhou, Bluhm, Christen and Shao-Horn 14 , Reference Gadre, Lee and Morgan 15 , Reference Feng, Yacoby, Gadre, Lee, Hong, Zhou, Biegalski, Christen, Adler, Morgan and Shao-Horn 20 ] In addition, the enhanced activity of LSC113 may also be attributed to the stabilized LSC113 surface by LSC214 phase, which suppresses the formation of Sr-enriched secondary particles on the LSC113 surface after a long-time annealing.[ Reference Lee, Lee, Hong, Biegalski, Morgan and Shao-Horn 17 ] However, the heterostructured oxide interfaces formed by decorating LSC214 on LSCF113 perovskites have shown negligible enhancement (up to two times) of the oxygen surface exchange kinetics of LSCF113,[ Reference Lee, Lee, Hong, Biegalski, Morgan and Shao-Horn 17 ] which can be attributed to no further increase in Sr concentration at the surface of LSCF113 induced by LSC214 decoration. While growing a more Sr-rich LSC214 on LSCF113 might yield enhancement due to the high oxygen surface exchange kinetics of LSC214 (x Sr > 1.0),[ Reference Gadre, Lee and Morgan 15 , Reference Nitadori, Muramatsu and Misono 21 ] such an approach is inhibited by difficulties in the synthesis of RP phase with high Sr substitution.[ Reference Sase, Hermes, Nakamura, Yashiro, Sato, Mizusaki, Kawada, Sakai, Yamaji, Horita, Yokokawa, Eguchi, Singhai, Yokokawa and Mizusaki 22 , Reference Shinomori, Kawasaki and Tokura 23 ]
In this study, we have developed the heterostructured oxide decoration on LSCF113, which leads to the enhancement of the surface activity of the LSCF113. Utilizing pulsed laser deposition (PLD), we employ two different types of surface decorations on the epitaxial LSCF113 thin films, which are the single-layer decoration of mixed LSC214 and LSC113 and the double-layer decoration of stacked LSC214 and LSC113. These structures stabilize the LSC113 phase, providing sufficient Sr sources and thermodynamic driving force for the Sr interdiffusion between LSC214 and LSC113. Electrochemical impedance spectroscopy (EIS) study reveals that the oxygen surface exchange coefficients (k i) of the LSCF113 thin films can be significantly enhanced up to ~1.5 orders of magnitudes higher than those of the undecorated LSCF113 by the heterostructured oxide interface engineering. In addition, the LSC113 with higher Sr content relative to the LSC214 single phase in both single-layer and double-layer decoration leads to higher enhancement in the surface exchange kinetics of the LSCF113, which suggests that the enhancement of the surface exchange kinetics of the LSCF113 can be attributed to an increase of Sr concentration on the multiphase heterostructured interface.
PLD was used to deposit the epitaxial ~65 nm LSCF113 thin films with the ~3 nm single-layer decoration of mixed LSC214 and LSC113 [Fig. 1(a)] and the double-layer decoration of stacked ~3 nm LSC214 and ~0.5 nm LSC113 [Fig. 1(b)] on an yttria-stabilized zirconia (YSZ) (001) substrate with a Gd-doped ceria (GDC) buffer layer. Out-of-plane x-ray diffraction (XRD) results [Figs. 1(c) and 1(d)] of the undecorated LSCF113, LSC214-decorated LSCF113, the LSCF113 with the single-layer decoration of mixed LSC214 and LSC113 thin films, and the LSCF113 with the double-layer decoration of stacked LSC214 and LSC113 thin films clearly show the presence of the (00l)pc (l is integer) peaks of LSCF113 and (00l)cubic (l is even) peaks of GDC and YSZ, indicating that the LSCF113 film was grown epitaxially with the following epitaxial relationships: (001)pcLSCF113//(001)cubicGDC//(001)cubicYSZ (where “pc” denotes the pseudocubic notation). With higher Sr content of LSC113 in the single- and double-layer decorations, the (00l)tetra. (l is the integer) peaks of LSC214 become visible, which represents (001)tetra.LSC214//(001)pcLSCF113//(001)cubicGDC//(001)cubicYSZ. The subscript “tetra.” denotes the tetragonal notation.[ Reference Lee, Grimaud, Crumlin, Mezghani, Habib, Feng, Hong, Biegalski, Christen and Shao-Horn 24 , Reference Lee, Lee, Grimaud, Hong, Biegalski, Morgan and Shao-Horn 25 ] Off-normal phi-scan analysis of the undecorated LSCF113 and LSC214-decorated LSCF113 films shows that LSC214 {103}tetra., LSCF113 {202}pc, GDC {202}cubic, and YSZ {202}cubic have strong peaks with fourfold cubic symmetry (Fig. S1†). This reveals the in-plane crystallographic relationships between GDC and YSZ (a cube-on-cube alignment), LSCF113 and GDC (an in-plane 45° rotation with [100]pcLSCF113//[110]cubicGDC//[110]cubicYSZ), and LSCF113 and LSC214 (no rotation with [100]pcLSCF113//[100]tetra.LSC214). Similar to our previous studies,[ Reference Crumlin, Ahn, Lee, Mutoro, Biegalski, Christen and Shao-Horn 13 , Reference Feng, Crumlin, Hong, Lee, Mutoro, Biegalski, Zhou, Bluhm, Christen and Shao-Horn 14 , Reference Lee, Lee, Hong, Biegalski, Morgan and Shao-Horn 17 , Reference Crumlin, Mutoro, Ahn, la O, Leonard, Borisevich, Biegalski, Christen and Shao-Horn 19 ] the relaxed lattice parameters, â of the epitaxial LSCF113 films with and without surface decoration in this study at room temperature did not change significantly, ranging from 3.898–3.904 Å (Table S1†). As shown in Table S1†, both in-plane and out-of-plane strains of LSCF113 films were not strongly influenced by the surface decoration, which is supported by the fact that the lattice constant of LSC214 (a tetra. ≈ 3.819 Å for LSC214 bulk[ Reference James, Tedesco, Cassidy, Colella and Smythe 26 ]) is very close to that of LSCF113 (a pc ≈ 3.885 Å for the LSCF113 bulk[ Reference Tai, Nasrallah, Anderson, Sparlin and Sehlin 27 ]) and LSC113 (a pc ≈ 3.854 Å for the LSC113 bulk[ Reference van Doorn and Burggraaf 28 ]). This observation is further supported by our recent work,[ Reference Feng, Crumlin, Hong, Lee, Mutoro, Biegalski, Zhou, Bluhm, Christen and Shao-Horn 14 ] where the LSC214 decoration has no influence on the in-plane and out-of-plane strains of the epitaxial LSC113 films at elevated temperatures. Details about deposition, lattice parameter calculation, and high-resolution XRD of LSC214-decorated LSC113 film can be found in the ESI†.

Figure 1. Schematic representation of (a) the LSCF113 with single-layer decoration of mixed LSC214 and LSC113, and (b) with double-layer decoration of stacked LSC214 and LSC113 epitaxial thin films. High-resolution XRD analysis of (c) the ~65 nm LSCF113 reference (green), the ~3 nm LSC214-decorated LSCF113 (yellow), and the LSCF113 with ~3 nm single-layer decorations of mixed LSC214 and LSC82113 (blue), LSC64113 (orange), and LSC46113 (red), and (d) the ~65 nm LSCF113 reference (green), the ~3 nm LSC214-decorated LSCF113 (yellow), and the LSCF113 with double-layer decorations of stacked ~3 nm LSC214 and ~0.5 nm LSC82113 (blue), ~0.5 nm LSC64113 (orange), and ~0.5 nm LSC46113 (red) epitaxial thin films on (001) YSZ substrates with GDC buffer layer. YSZ substrate and GDC peaks are indicated with pounds (#) and asterisks (*), respectively.
EIS results of geometrically well-defined microelectrodes (200 µm in diameter), measured at 550 °C are shown in Fig. 2. These microelectrodes were fabricated by photolithography and acid etched for the epitaxial LSCF113 thin films with LSC214 decoration and single-layer decorations of mixed LSC214 and three different Sr contents of LSC113 (Sr = 0.2, 0.4, and 0.6). The predominant semicircle was found to increase with decreasing oxygen partial pressure [Fig. 2(a)], where EIS data of all samples used in this study showed nearly perfect semicircle impedances.[ Reference Adler 6 ] Considering the fact that the film thicknesses are much smaller than the critical thickness for bulk transport limitation (estimated to 3.28 µm for bulk LSCF113 at 550 °C[ Reference Steele and Bae 29 ]), the oxygen partial pressure [p(O2)]-dependent impedance responses suggest that the oxygen surface exchange kinetics governs the oxygen electrocatalysis on the film surface. In Fig. 2(b), the real part of the impedance of the predominant semicircle decreased with increasing Sr content of LSC113 in the single-layer decoration of mixed LSC214 and LSC113, where the oxygen surface exchange coefficient (kq ) of the LSCF113 with mixed LSC214 and La0.4Sr0.6CoO3−δ (LSC46113) decoration was found to be ~7 times higher than that of undecorated LSCF113 and LSC214-decorated LSCF113. This observation indicates that higher Sr content in mixed LSC214 and LSC113 decoration can lead to higher surface exchange kinetics of the LSCF113.

Figure 2. EIS results of microelectrodes (200 µm in diameter) for the epitaxial LSCF113 thin films with LSC214 decoration, and single-layer decorations of mixed LSC214 and LSC113 on YSZ (001) with a GDC buffer layer at 550 °C. (a) Nyquist plot at 550 °C as a function of oxygen partial pressure, p (02), of the LSCF113 thin films with single-layer decoration of mixed LSC214 and LSC46113. (b) Nyquist plot at 550 °C with an 1 atm of p(02) of the LSCF113 (green), the LSC214-decorated LSCF113 (yellow), and the LSCF113 with ~3 nm single-layer decoration of mixed LSC214 and LSC82113 (blue), LSC64113 (orange), and LSC46113 (red) thin films. (c) p(02) dependency of the surface exchange coefficients (k < l) of the LSCF113 (green), the LSC214-decorated LSCF113 (yellow), and the LSCF113 with ~3 nm single-layer decoration of mixed LSC214 and LSC82113 (blue), LSC64113 (orange), and LSC46113 (red) thin films. All EIS spectra were collected at 550 °C.
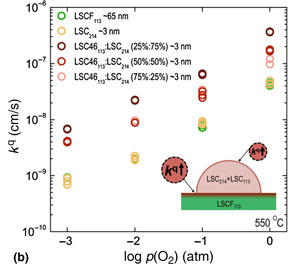
To further investigate the effect of Sr concentration in the mixed LSC214 and LSC113 phase on the surface exchange kinetics of the LSCF113, a different ratio between LSC214 and LSC46113 was applied for decorating the surface of the LSCF113. EIS data collected from the LSCF113 with and without the single-layer decoration of mixed LSC214 and LSC46113 thin films at 550 °C with an p(O2) of 1 atm is shown in Fig. 3(a). It is noted that the kq values of the LSCF113 with 75% of LSC214 and 25% of LSC46113 decoration were found to be ~1.1 orders of magnitude higher than those of the LSCF113 with and without LSC214 decoration, as shown in Fig. 3(b). To understand these changes we consider if the decorations may lead to the enhancement of Sr in the LSCF113 surface, which would be expected to increase the oxygen 2p band center relative to the Fermi level,[ Reference Lee, Lee, Hong, Biegalski, Morgan and Shao-Horn 17 ] which in turn is expected to correlate with the enhancement of the oxygen surface exchange kinetics.[ Reference Lee, Lee, Hong, Biegalski, Morgan and Shao-Horn 17 , Reference Feng, Yacoby, Gadre, Lee, Hong, Zhou, Biegalski, Christen, Adler, Morgan and Shao-Horn 20 , Reference Lee, Kleis, Rossmeisl, Shao-Horn and Morgan 30 ] In the case of the LSC214 decorated LSCF113, it has been proposed that low enhancement is observed because there is a negligible change of the surface Sr concentration at the heterostructured interface due to the initially high Sr surface concentration (~100%) of the stable LSCF113 (001) surface. This high Sr concentration cannot be easily increased. In contrast, the addition of the LSC113 phase into the LSC214 can provide the increased Sr content in LSC214 and associated thermodynamic driving force for Sr interdiffusion from the LSC113 into the LSC214. We propose that this driving force is large enough to result in higher Sr concentration in the surface decoration layer of mixed LSC214 and LSC113 on the LSCF113 surface. Accordingly, this Sr enrichment is expected to uplift the oxygen 2p band center (relative to the Fermi level) of the LSCF113 interface layer and enhance the oxygen exchange kinetics of the LSCF113, as reported previously.[ Reference Lee, Lee, Hong, Biegalski, Morgan and Shao-Horn 17 ] Interestingly, the enhancement in the surface exchange kinetics of the LSCF113 was found to decrease with increasing the LSC46113 ratio in the LSC214 phase. This can be explained by the fact that the LSC214 phase becomes unstable with increasing LSC46113, which can be supported by the reduced intensity of LSC214 (00l)tetra. peak in Fig. S2†. Although a detailed study of the electronic structure changes is needed, the enhanced Sr concentration in the LSC214 by mixing with LSC113 may be responsible for enhancing the surface exchange kinetics of the LSCF113.

Figure 3. EIS results of microelectrodes (200 µm in diameter) for the epitaxial LSCF113 thin films with LSC214 decoration, and single-layer decorations of mixed LSC214 and LSC46113 on YSZ (001) with a GDC buffer layer at 550 °C. (a) Nyquist plot at 550 °C with an 1 atm of p(02) of the LSCF113 (green), the LSC214-decorated LSCF113 (yellow), and the LSCF113 with ~3 nm single-layer decoration of mixed LSC214 and LSC64113 (75%:25%) (dark red), LSC214 and LSC64113 (50%:50%) (red), and LSC214 and LSC64113 (25%:75%) (light red) thin films. (b) p(02) dependency of kG calculated from EIS spectra collected at 550 °C of the LSCF113 (green), the LSC214-decorated LSCF113 (yellow), and the LSCF113 with ~3 nm single-layer decoration of mixed LSC214 and LSC64113 (75%:25%) (dark red), LSC214 and LSC64113 (50%:50%) (red), and LSC214 and LSC64113 (25%:75%) (light red) thin films. Inset shows a hypothetical model: enhancement of the Sr content at the top surface of the LSCF113 due to adding LSC113 to LSC214.
Figure 4(b) shows the kq values of the LSCF113 with double-layer decoration of stacked LSC214 and three different Sr contents of LSC113 (LSC82113, LSC64113, and LSC46113) thin films, extracted from the EIS data [Fig. 4(a)]. As shown in Fig. 4(b), the kq values of the LSCF113 thin films were found to change with the additional LSC113 phase between the LSCF113 and LSC214, which can be attributed to a change in the Sr concentration at the multiphase heterostructured interface. We hypothesize that the added LSC113 phase provides sufficient Sr sources for surface Sr redistribution between LSC113 and LSC214, which results in increased Sr segregation on the LSCF interface layer. This hypothesis is consistent with our previous ab initio DFT calculations,[ Reference Lee, Lee, Hong, Biegalski, Morgan and Shao-Horn 17 ] which found that the thermodynamic driving force for Sr interdiffusion from La0.625Sr0.375Co0.25Fe0.75O3 to (La0.5Sr0.5)2CoO4 (−0.12 eV) is much weaker than that from La0.75Sr0.25CoO3 to (La0.5Sr0.5)2CoO4 (−0.7 eV). This driving force is likely responsible for different enhancements in the surface Sr content in the LSCF113 films upon LSC214 decoration, resulting in different surface exchange kinetics.

Figure 4. EIS results of microelectrodes (200 µm in diameter) for the epitaxial LSCF113 thin films with LSC214 decoration, and double-layer decorations of stacked LSC214 and LSC113 on YSZ (001) with a GDC buffer layer at 550 °C. (a) Nyquist plot at 550 °C with an 1 atm of p(02) of the LSCF113 (green), the LSC214-decorated LSCF113 (yellow), and the LSCF113 with double-layer decoration of stacked ~3 nm LSC214 and ~0.5 nm LSC82113 (blue), ~0.5 nm LSC64113 (orange), and ~0.5 nm LSC46113 (red) thin films. (b) p(02) of the kq calculated from EIS spectra collected at 550 °C of the LSCF113 (green), the LSC214-decorated LSCF113 (yellow), and the LSCF113 with double-layer decoration of stacked ~3 nm LSC214 and ~0.5 nm LSC82113 (blue), ~0.5 nm LSC64113 (orange), and ~0.5 nm LSC46113 (red) thin films. Inset shows a hypothetical model: enhancement of the Sr content at the interface between the LSCF113 and the LSC214 phase due to an increase in the Sr interdiffusion from LSC113 to LSC214.
LSCF113 with the double-layer decoration of stacked LSC214 and LSC46113 shows significantly higher kq values up to ~1.5 orders of magnitude relative to the undecorated LSCF113 and LSC214-decorated LSCF113. The enhancement can be attributed to Sr segregation at the interface between LSC214 and LSC46113 and on the LSC214 surface at the expense of Sr in LSC46113 in the double-layer decoration considering markedly enhanced activity of LSC214-decorated LSC82113 in the previous work.[ Reference Lee, Lee, Hong, Biegalski, Morgan and Shao-Horn 17 ]
In conclusion, we demonstrate that the oxygen surface exchange kinetics of the (001)-oriented epitaxial LSCF113 thin films can be markedly improved by the advanced heterostructured oxide interface engineering using the single-layer decoration of mixed LSC214 and LSC113 and double-layer decoration of stacked LSC214 and LSC113. This result extends previous results,[ Reference Lee, Lee, Hong, Biegalski, Morgan and Shao-Horn 17 ] showing enhancement from decoration of LSC214 on LSC113 to the LSCF113 material, which is of significantly more interest for commercial applications than LSC113. The oxygen surface exchange coefficients of the LSCF113 with single-layer decoration of mixed LSC214 and LSC113 are ~1.1 orders of magnitude greater than those of the undecorated LSCF113 and LSC214-decorated LSCF113. In addition, the oxygen surface exchange coefficients of the LSCF113 with double layer decoration of stacked LSC214 and LSC113 are ~1.5 orders of magnitude higher than those of the undecorated LSCF113 with and without LSC214 decoration. The previous work[ Reference Lee, Lee, Hong, Biegalski, Morgan and Shao-Horn 17 ] suggests a strong correlation between the O 2p band center and surface exchange kinetics, where surface Sr segregation in the perovskite structure and associated O 2p band uplift could increase the surface exchange rate. Therefore, we hypothesize that the decoration on the surface of LSCF113 provides Sr segregation at the interface LSC214 and LSC113 and on the surface of LSC214, which can uplift in the position of the O 2p band center relative to Fermi energy of the LSCF interface layer in comparison to that of the LSCF113 surface. This work illustrates that heterostructured oxide interface engineering is a strategy, which can enhance multiple types of active oxide materials. Such approaches could potentially be utilized in the infiltration process for decorating cathodes to enhance the performance of SOFCs.
Supplementary material
The supplementary material for this article can be found at http://dx.doi.org/10.1557/mrc.2016.28
Acknowledgments
This work was supported by the Department of Energy (DOE), National Energy Technology Laboratory (NETL), Solid State Energy Conversion Alliance (SECA) Core Technology Program (Funding Opportunity Number DEFE0009435) and the Skoltech-MIT Center for Electrochemical Energy. The PLD and XRD performed were conducted at the Center for Nanophase Materials Sciences, which is sponsored at Oak Ridge National Laboratory by the Scientific User Facilities Division, Office of Basic Energy Sciences, U.S. Department of Energy, and computations in this work were also benefited from the use of the National Energy Research Scientific Computing Center allocation of the Center for Nanophase Materials Sciences at Oak Ridge National Laboratory, both under grant number CNMS2013-292.