Article contents
Equilibrium ternary intermetallic phase in the Mg–Zn–Ca system
Published online by Cambridge University Press: 17 June 2016
Abstract
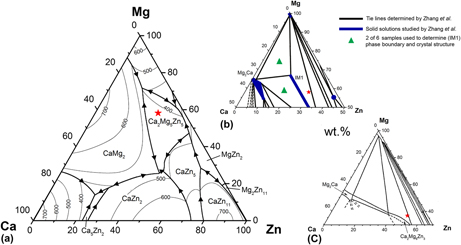
This study investigates the ternary intermetallic phases in the Mg–Zn–Ca system, which is of great interest for metallic biodegradable implant applications. According to published phase diagrams, the key alloy composition studied herein is located within the Ca2Mg5Zn5, Ca2Mg6Zn3, and IM1 phase fields. Through controlled cooling of the melt, a quasibinary ∼Ca2Mg5Zn5–Mg microstructure was obtained. The large polygonal grains had a composition of Ca2Mg5Zn5 as determined by energy-dispersive x-ray spectroscopy (EDX). Differential scanning calorimetry revealed that Ca2Mg5Zn5 begins to form at ∼417 °C, and the eutectic temperature is ∼369 °C. Based on single-crystal x-ray diffraction data, Ca2Mg5Zn5 was determined to be hexagonal (P63/mmc), with lattice parameters of a = 9.5949(3) Å and c = 10.0344(3) Å. This was also verified by transmission electron microscopy. Further refinements, which considered the possibility of mixed Mg/Zn sites, significantly improved the data fit compared to the initial ordered structural model. The final refined structure possesses a composition of Ca16Mg42Zn42, very similar to the chemical analysis results from EDX.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2016
Footnotes
This author was an editor of this journal during the review and decision stage. For the JMR policy on review and publication of manuscripts authored by editors, please refer to http://www.mrs.org/jmr-editor-manuscripts/.
Contributing Editor: Yang-T. Cheng
References
REFERENCES
- 11
- Cited by




