TTTS is a severe condition complicating about 10% of monochorionic–diamniotic twin pregnancies (Lewi et al., Reference Lewi, Jani, Blickstein, Huber, Gucciardo, Van Mieghem and Deprest2008). Laser surgery is the optimal treatment for severe TTTS. Although the neonatal outcome has significantly improved after introduction of laser surgery, several complications are reported, such as PPROM, clinical, or histologic chorioamnionitis, recurrent or reversed TTTS, and post-laser twin anemia-polycythemia sequence (TAPS). PPROM is partly due to the specific nature of the fetoscopic intervention itself, in which a fetoscope is introduced into the amniotic cavity causing iatrogenic PPROM (Chalouhi et al., Reference Chalouhi, Essaoui, Stirnemann, Quibel, Deloison, Salomon and Ville2011; Roberts et al., Reference Roberts, Neilson, Kilby and Gates2014; Salomon et al., Reference Salomon, Ortqvist, Aegerter, Bussieres, Staracci, Stirnemann and Ville2010; Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004; Yamamoto et al., Reference Yamamoto, El Murr, Robyr, Leleu, Takahashi and Ville2005). Previous studies also demonstrated that the risk of chorioamnionitis and EOS is increased after invasive intrauterine procedures, such as amniocentesis or fetal blood sampling, in both singletons and twins (André et al., Reference André, Thébaud, Guibert, Audibert, Lacaze-Masmonteil and Dehan2000; Brambati et al., Reference Brambati, Lanzani and Tului1990; d'Ercole et al., Reference d'Ercole, Shojai, Desbriere, Chau, Bretelle, Piéchon and Bouli2003; Hamoda & Chamberlain, Reference Hamoda and Chamberlain2002; Kim et al., Reference Kim, Romero, Chaemsaithong, Chaiyasit, Yoon and Kim2015; Soman et al., Reference Soman, Green and Daling1985; Workman & Philpott-Howard, Reference Workman and Philpott-Howard1997). EOS is usually caused by bacterial pathogens, most commonly group B streptococcus or Escherichia coli, transmitted vertically from mother to infant before or during delivery, and is one of the leading causes of neonatal mortality and morbidity (Naeye et al., Reference Naeye, Dellinger and Blanc1971; Stoll et al., Reference Stoll, Hansen, Sánchez, Faix, Poindexter, Van Meurs and Higgins2011; Weston et al., Reference Weston, Pondo, Lewis, Martell-Cleary, Morin, Jewell and Schrag2011). The risk of EOS following laser surgery for TTTS has, however, not yet been studied. The purpose of this study was to investigate the occurrence and risk factors of EOS after laser surgery for the treatment of TTTS.
Materials and Methods
Design and Study Population
All consecutive monochorionic twins delivered at the Leiden University Medical Center between March 2002 and April 2015 were eligible for this study. We included all monochorionic twins with TTTS treated with laser surgery (TTTS group) and compared them with a control group of uncomplicated monochorionic twins (no-TTTS group). Monochorionic twins with TTTS not managed with laser surgery and TAPS cases were excluded. We also excluded monochorionic twins with single or double fetal demise. These twins had to be excluded since sepsis workup was not performed, preventing the diagnosis of EOS. TTTS was diagnosed according to the Eurofetus criteria, defined as a maximal vertical pocket (MVP) of <2 cm in the donor combined with a MVP of >8 cm in the recipient before 20 weeks’ gestation and >10 cm thereafter (Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004). Staging of TTTS was defined according to Quintero's system (Quintero et al., Reference Quintero, Morales, Allen, Bornick, Johnson and Kruger1999). The procedure of laser surgery for TTTS was published previously (Slaghekke, Lewi et al., Reference Slaghekke, Lewi, Middeldorp, Weingertner, Klumper, Dekonick and Lopriore2014). Since 2008, the Solomon laser technique was introduced to reduce the risk of residual anastomoses and associated post-operation complications (Slaghekke, Lopriore et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014). We thus divided the study period into two groups, that is, the first group from 2002 to 2008 (first study period) and the second group from 2009 to 2015 (second study period). Diagnosis of TAPS was based on previously published international criteria (Slaghekke et al., Reference Slaghekke, Kist, Oepkes, Pasman, Middeldorp, Klumper and Lopriore2010).
Outcome Variables
The following perinatal variables were recorded, including gestational age at laser surgery, study period (first study period: 2002–2008; second study period: 2009–2015), placental localization, gestational age at birth, birth weight and birth weight discordance, mode of delivery, maternal fever (≥38 °C), use of epidural anesthetics during labor, and PPROM (<37 weeks gestational age for >24 hours). Data from maternal vaginal or urinary cultures were mostly not available and therefore not recorded. After birth, routine blood tests were performed in all preterm neonates that were born before the gestational age of 37 weeks. C-reactive protein (CRP) levels and immature/total neutrophil ratios (I/T ratio) were documented within the first 72 hours after birth. Elevated CRP was defined as a level >20 mg/L. An I/T ratio >0.22 was suggestive of infection (Polin, Reference Polin2012). Blood cultures were performed within 72 hours after birth in all children with suspected perinatal infection. Diagnosis of EOS was based on a positive blood culture within the first 72 hours postpartum (proven EOS) or the necessity for administration of a full course (5–7 days) of antibiotics due to perinatal risk factors and/or clinical signs of neonatal infection, though without a positive blood culture (suspected EOS). Blood cultures were evaluated for the chance of contamination. When a coagulase negative staphylococcus was found, these were only considered proven EOS if there were clinical and/or laboratory signs (CRP >20 mg/L or I/T ratio >0.22) of infection. Risk factors for perinatal infection included one or more of the following items: maternal group B streptococcal colonization, maternal bacteriuria or infection, preterm birth (gestational age <37 weeks) following spontaneous labor, ruptured membranes >24 hours in preterm birth, maternal fever (≥38 °C) or parenteral antibiotics given to the mother for (suspected) invasive bacterial infection (Dutch Society of Obstetrics and Gynaecology, 2012). The primary outcome was the occurrence of proven and/or suspected EOS. Secondary outcomes were CRP and I/T ratio in the first 72 hours after birth. Potential risk factors for EOS that were studied included gestational age at laser surgery, gestational age at birth, interval between laser surgery and birth, anterior placenta, study period (first study period: 2002–2008; second study period: 2009–2015), and PPROM.
Statistical Analysis
The Kolmogorov–Smirnov test was used to assess the normality of continuous variables. Student's t-test or the Mann–Whitney test were applied to analyze continuous variables, where appropriate. A chi-square test or Fisher exact test was used to compare dichotomous data. Paired variables were compared using the McNemar or Wilcoxon signed rank test, if applicable. The results were displayed as median and interquartile range (IQR), mean and range or number and percentage, where appropriate. The potential risk factors for EOS were analyzed in a univariable regression model (generalized estimating equation). The factors significantly associated with EOS in the univariate analysis were further used to build a multivariable regression model (generalized estimating equation) to assess their independence in relation to EOS, because pair-wised comparison in twins is possible in this binominal model. The results of correlation and risk factor analysis were displayed as OR and 95% CI. Statistical significance was assumed if p < .05 in all analyses, besides the univariate analysis (p < .10). Statistical analysis was performed using IBM SPSS Statistics 22.0® (IBM Corporation, Armonk, New York, USA).
Results
During the study period, 479 eligible twin pairs with two live-born neonates were delivered at our hospital and included in the study, of which 208 (43%) were in the TTTS group after laser surgery and 271 (57%) in the no-TTTS group. The baseline characteristics of the two study groups are summarized in Table 1. In the TTTS group, 14% (28/208) of the twin pairs were Quintero stage 1, 32% (67/208) stage 2, 51% (105/208) stage 3, and 4% (8/208) stage 4. Median gestational age at laser surgery was 20 weeks (IQR 18–23 weeks). In the no-TTTS group, selective fetal growth restriction occurred in 65/271 (24%) twin pairs, and 14/271 (5%) twin pairs were monoamniotic.
TABLE 1 Demographics and Baseline Characteristics
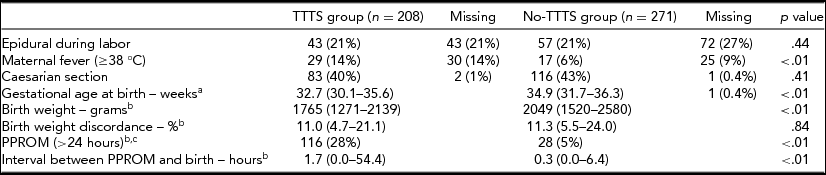
Data is displayed as median (IQR) or number (%). aIn the TTTS group the gestational age at birth was known in all neonates. bFor these variables the data were complete. cDenotes the number of fetus instead of twin pairs.
A comparison of primary and secondary outcomes between the TTTS group and no-TTTS group is shown in Table 2. The rate of proven EOS was 2% (10/416) in the TTTS group and 0.4% (2/542) in the no-TTTS group (relative ratio [RR] 6.67, 95% CI 1.45–30.59, p = .02). The rate of suspected EOS was 14% (58/416) in the TTTS group and 10% (53/542) in the no-TTTS group (RR 1.50, 95% CI 1.01–2.24, p = .04). The overall rate of EOS (proven or suspected) was significantly higher in the TTTS group compared to the no-TTTS group, 16% versus 10% respectively (RR 1.74, 95% CI 1.19–2.55, p < .01). In the TTTS group after laser surgery, CRP and I/T ratio were also significantly increased compared to the no-TTTS group (both p < .01). After controlling for gestational age at birth, no difference in suspected EOS was detected in the TTTS group compared to the no-TTTS group (OR 0.95, 95% CI 0.62–1.46, p = .82), but the OR of proven EOS was still increased (OR 5.03, 95% CI 1.08–23.54, p = .04).
TABLE 2 Primary and Secondary Outcomes in the TTTS Group and Control Group
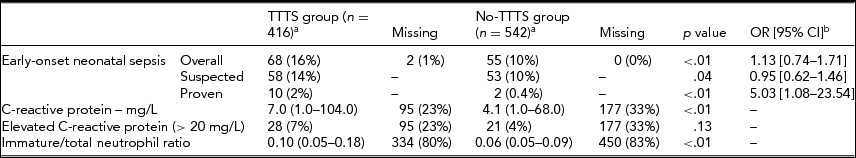
Data is displayed as median (IQR) or number (%). C-reactive protein is displayed as mean (range). aDenotes the number of fetus instead of twin pairs. bCorrected for gestational age at birth.
Analysis between ex-donors and ex-recipients in the TTTS group showed no difference in suspected or proven EOS (Table 3). However, the occurrence of PPROM was significantly higher in ex-recipients than in ex-donors, 69/206 (34%) versus 46/206 (22%) respectively (RR 1.75, 95% CI 1.13–2.71 p = .01). Overall, EOS occurred in 62% (42/68) of cases in both neonates within the same twin pair and in only one neonate of the twin pair in the remaining 38% (26/68) of EOS cases.
TABLE 3 Comparison Between the Ex-Donor and the Ex-Recipient Twins in TTTS Managed with Laser Surgery
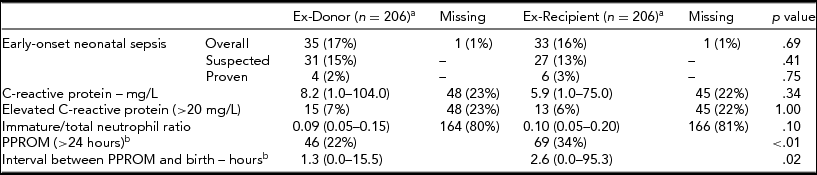
Data is displayed as median (IQR) or number (%). C-reactive protein is displayed as mean (range). aFor two twin pairs the donor and recipient were unknown. bFor these variables the data were complete.
Univariate analysis of potential risk factors for the occurrence of EOS in TTTS managed with laser surgery showed that EOS was associated with lower gestational age at birth (OR 0.75, 95% CI 0.67–0.83, p < .01), shorter interval between laser surgery and birth (OR 0.89, 95% CI 0.84–0.95, p < .01), anterior placenta (OR 1.89, 95% CI 0.99–3.63, p = .06), first study period (OR 2.32, 95% CI 1.20–4.47, p = .01), and PPROM (OR 3.74, 95% CI 2.06–6.81, p < .01). After multivariate analysis, only lower gestational age at birth (OR 0.75, 95% CI 0.63–0.88, p < .01), first study period (OR 2.25, 95% CI 1.08–4.67, p = .03) and PPROM (OR 2.47, 95% CI 1.28–4.75, p < .01) were independently associated with the occurrence of EOS (Table 4).
TABLE 4 Analysis of Risk Factors for Early-Onset Neonatal Sepsis in TTTS Group Treated with Laser Surgery

Data is displayed as median (IQR) or number (%).
In the group of neonates with proven EOS in the TTTS group, 60% (6/10) of infections was due to infection with gram positive bacteria (coagulase negative staphylococcus, n = 4 with elevated CRP 13, 101, 68, 75 mg/L, respectively; Staphylococcus aureus, n = 1, Enterococcus faecalis, n = 1) and 40% (4/10) of infections was due to gram negative bacteria (Escherichia coli, n = 2, Klebsiella pneumoniae, n = 2). In the two cases with proven EOS in the control group, infection was due in both cases to coagulase negative staphylococcus, with CRP 33 and 67 mg/L, respectively.
Discussion
This is the first study to investigate the occurrence of EOS following laser surgery for the treatment of TTTS. Our findings show that the rate of EOS in TTTS treated with laser surgery is slightly increased compared to monochorionic twins without invasive fetoscopic intervention. Risk factor analysis shows that EOS after laser surgery for TTTS is independently associated with lower gestational age at birth, first study period, and PPROM.
Small studies have linked invasive intrauterine procedures, such as chorionic villus sampling, amniocentesis and fetal blood sampling, to the occurrence of EOS (André et al., Reference André, Thébaud, Guibert, Audibert, Lacaze-Masmonteil and Dehan2000; Brambati et al., Reference Brambati, Lanzani and Tului1990; d'Ercole et al., Reference d'Ercole, Shojai, Desbriere, Chau, Bretelle, Piéchon and Bouli2003; Hamoda & Chamberlain, Reference Hamoda and Chamberlain2002; Kim et al., Reference Kim, Romero, Chaemsaithong, Chaiyasit, Yoon and Kim2015; Soman et al., Reference Soman, Green and Daling1985; Workman & Philpott-Howard, Reference Workman and Philpott-Howard1997). Reports on the frequency of EOS after laser surgery are, however, lacking. EOS is usually caused by bacterial pathogens transmitted vertically from mother to the fetus perinatally, resulting in intrauterine infection and ensuing EOS (Naeye et al., Reference Naeye, Dellinger and Blanc1971). Previous studies have suggested a possible association between laser surgery and increased risk of chorioamnionitis (Habli et al., Reference Habli, Bombrys, Lewis, Lim, Polzin, Maxwell and Crombleholme2009; Meriki et al., Reference Meriki, Smoleniec, Challis and Welsh2010; Merz et al., Reference Merz, Tchatcheva, Gembruch and Kohl2010; Rossi et al., Reference Rossi, Kaufman, Bornick and Quintero2008; Rustico et al., Reference Rustico, Lanna, Faiola, Schena, Dell'avanzo, Mantegazza and Ferrazzi2012; Stirnemann et al., Reference Stirnemann, Quibel, Essaoui, Salomon, Bussieres and Ville2012; Yamamoto et al., Reference Yamamoto, El Murr, Robyr, Leleu, Takahashi and Ville2005). In a cohort of 266 TTTS cases treated with laser surgery, Rossi et al. (Reference Rossi, Kaufman, Bornick and Quintero2008) reported that the risk of iatrogenic PPROM and clinical chorioamnionitis was 38% and 0%, respectively. However, the diagnosis of chorioamnionitis was not based on histopathological evaluation, and the risk of neonatal EOS was not reported. In another larger cohort of 602 TTTS cases treated with laser surgery, Stirnemann et al. (Reference Stirnemann, Quibel, Essaoui, Salomon, Bussieres and Ville2012) reported a 4% incidence of clinical chorioamnionitis, but again, data on neonatal EOS were not described. In a prospective study, Lewi et al. (Reference Lewi, Jani, Blickstein, Huber, Gucciardo, Van Mieghem and Deprest2008) reported a rate of neonatal sepsis of 4% in monochorionic twin pregnancies. However, the frequency of neonatal sepsis in the subgroup of TTTS managed with laser surgery was not specified, and the definition of neonatal sepsis was not clearly described. In our study, we found that the rate of proven EOS was 2% in monochorionic twins managed with laser surgery and 0.4% in monochorionic twins not managed with laser surgery. Laboratory investigations also showed higher CRP levels and I/T ratio in the TTTS group. Whether these result from bacterial infection or post-operative inflammation after laser surgery is not clear.
This study further analyzed the association between several perinatal variables with the occurrence of EOS in the subgroup of TTTS treated with laser surgery. We found that lower gestational age at birth, first study period, and PPROM were independently associated with EOS after laser surgery. Iatrogenic PPROM is a known risk associated with invasive intrauterine procedures. In accordance with previous studies, we also found an increased risk of PPROM (28%) after laser surgery (Rossi et al., Reference Rossi, Kaufman, Bornick and Quintero2008; Yamamoto et al., Reference Yamamoto, El Murr, Robyr, Leleu, Takahashi and Ville2005). PPROM may facilitate the invasion of microorganisms into the amniotic cavity, leading to histopathological and/or clinical chorioamnionitis and resulting in fetuses exposed to high risk of infection (Kim et al., Reference Kim, Romero, Chaemsaithong, Chaiyasit, Yoon and Kim2015; Wortham et al., Reference Wortham, Hansen, Schrag, Hale, Van Meurs, Sánchez and Stoll2016). PPROM may also lead to premature delivery, which is a known risk factor for both EOS as well as late onset sepsis (Martius et al., Reference Martius, Roos, Gora, Oehler, Schrod, Papadopoulos and Gross1999; Schrag et al., Reference Schrag, Hadler, Arnold, Martell-Cleary, Reingold and Schuchat2006; Schuchat et al., Reference Schuchat, Zywicki, Dinsmoor, Mercer, Romaguera, O'Sullivan and Levine2000). In addition, in neonates with lower gestational age at birth, the innate, and adaptive immunity may be more immature, increasing the risk of infection (Wynn & Levy, Reference Wynn and Levy2010). Lastly, we also found an increased risk of EOS in the first study period. The decrease in risk of infection in the second, most recent study period might be a direct effect of the learning curve related to laser surgery. Increased experience and improved laser technique within recent years may result in a reduction of procedure-related complications (Peeters et al., Reference Peeters, Van Zwet, Oepkes, Lopriore, Klumper and Middeldorp2014).
Univariate analysis in this study also showed that a shorter interval between laser surgery and birth and anterior placenta are associated with EOS. However, after correction in multivariate analysis, they were no longer associated with the occurrence of EOS. A shorter interval between laser surgery and birth might be a result of PPROM and chorioamnionitis and result in premature delivery. Anterior placenta increases the complexity of the fetal surgical intervention and may thus increase the risk of perinatal infection due to a longer operation time and a higher risk of complications such as iatrogenic PPROM (Yamamoto et al., Reference Yamamoto, El Murr, Robyr, Leleu, Takahashi and Ville2005). This hypothesis should be further explored in other cohorts.
An important asset of this study is that it is a large prospective cohort study, including monochorionic twins with and without TTTS. Furthermore, it is the first study to investigate the occurrence of EOS after laser surgery for TTTS, an important evaluation of an invasive procedure, which is increasingly being used throughout the world. However, our study has several limitations for which the results should be carefully interpreted. An important limitation is that histopathological evaluation of the placenta was not routinely performed. In addition, by excluding monochorionic twin pregnancies with fetal demise from this study, we may have introduced a selection bias. However, these cases had to be excluded since sepsis workup was not performed in these neonates, preventing diagnosis of EOS. If demise in these cases was due to perinatal infection, the rate of EOS in the TTTS group would have been higher and our data may underestimate the true occurrence of EOS. On the other hand, neonatal infection in some cases in the TTTS group could be the result of prematurity due to immature immune system and not a direct result of the laser surgery. Therefore, our data may also overestimate the true risk of infection after laser. However, studying this aspect is not considered ethical, since the majority of TTTS is currently managed with laser surgery (Roberts et al., Reference Roberts, Neilson, Kilby and Gates2014; Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004).
Conclusions
The occurrence of EOS in TTTS managed with laser surgery is low, but increased compared to uncomplicated monochorionic twins. Future studies and developments focusing on improvement of laser technique and instruments may reduce the risk of (iatrogenic) PPROM, chorioamnionitis, and perinatal sepsis. Neonatologists should be aware of the risk of EOS when caring for newborns treated with laser surgery for TTTS during pregnancy.








