The placenta in monochorionic twins is unique. Both fetuses share a single placenta and vascular anastomoses on the fetal surface connect the blood circulations of both twins. Through these anastomoses, there is a continuous exchange of blood between the twins. Imbalances in this intertwin exchange are often the cause of severe pathology, such as TTTS or TAPS (Lewi et al., Reference Lewi, Deprest and Hecher2013). In this overview, we hope to improve the understanding of the role of the placenta in these two conditions.
The Placenta in TTTS
The diagnosis of TTTS is based on the presence of polyhydramnios in the recipient twin, with a distended bladder and oliguric oligohydramnios (deepest vertical pool [DVP] ≤2 cm) in the donor twin, who usually has a small or empty bladder (Quintero et al., Reference Quintero, Morales, Allen, Bornick, Johnson and Kruger1999). Polyhydramnios is defined as a DVP of ≥8 cm, although in Europe a cut-off DVP of ≥10 cm is used after 20 weeks to adjust for the increase in amniotic fluid with gestation (Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004). TTTS occurs in 10% of monochorionic twins and presents typically in the previable period, between 16 and 26 weeks (Lewi et al., Reference Lewi, Gucciardo, Huber, Jani, Van Mieghem, Doné and Deprest2008).
The underlying cause appears to be a transfusion of blood from the donor twin to the recipient twin through placental vascular anastomoses. A randomized controlled trial demonstrated that laser coagulation of the placental anastomoses is the best treatment for the condition (Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004). Laser surgery effectively resolves the syndrome (Lewi et al., Reference Lewi, Jani, Cannie, Robyr, Ville, Hecher and Deprest2006). However, this does not explain the whole clinical picture, as there is usually no hemoglobin discordance between the fetuses in the classic form of TTTS (Saunders et al., Reference Saunders, Snijders and Nicolaides1991). The donor twin is volume-depleted and the recipient twin is hypervolemic, suggesting a volume shift to be the predominant part of the syndrome. Furthermore, the recipient twin is at risk of cardiomyopathy and cardiac failure, which cannot be fully explained by volume overload alone. We know that renin is upregulated in the donor twin and downregulated in the recipient twin. Through the shared circulation, however, both twins are probably exposed to the same high renin levels in utero. This may contribute to hypertension and cardiomyopathy in the recipient twin (Mahieu-Caputo et al., Reference Mahieu-Caputo, Meulemans, Martinovic, Gubler, Delezoide, Muller and Dommergues2005). Concentrations of brain natriuretic peptide and endothelin-1 — involved in fluid homeostasis — are higher in the amniotic fluid of recipient twins compared to donor twins (Bajoria et al., Reference Bajoria, Ward and Chatterjee2003). Most likely, several other unidentified hormonal factors play a role in the pathogenesis of TTTS.
Anastomoses in TTTS Placentas
The anastomoses in a TTTS placenta are different from the uncomplicated monochorionic placentas. There are three types of anastomoses: artery-to-artery (AA), vein-to-vein (VV) and artery-to-vein (AV) anastomoses. AA and VV anastomoses form direct communications on the surface of the chorionic plate and are bidirectional. Artery-to-vein (AV) anastomoses are located deep in the placental tissue and are obligate unidirectional. The AV anastomosis itself occurs at a capillary level deep in the shared placental lobule. AV anastomoses always direct flow from one twin to the other, while AA and VV anastomoses allow flow in both directions, depending on intertwin pressure gradients. An AA anastomosis can function as an AV anastomosis from twin 1 to 2 as well as from twin 2 to 1 and is in fact a flexible AV anastomosis. The bidirectional AA anastomosis can thus compensate for the imbalanced flow through the unidirectional AV anastomoses (Lewi et al., Reference Lewi, Deprest and Hecher2013). Anastomoses are present in 94% of monochorionic placentas not complicated by TTTS. There is usually only one AA and/or VV anastomosis per placenta, while multiple AV anastomoses may be present (Lewi et al., Reference Lewi, Cannie, Blickstein, Jani, Huber, Hecher and Deprest2007); see Figure 1.

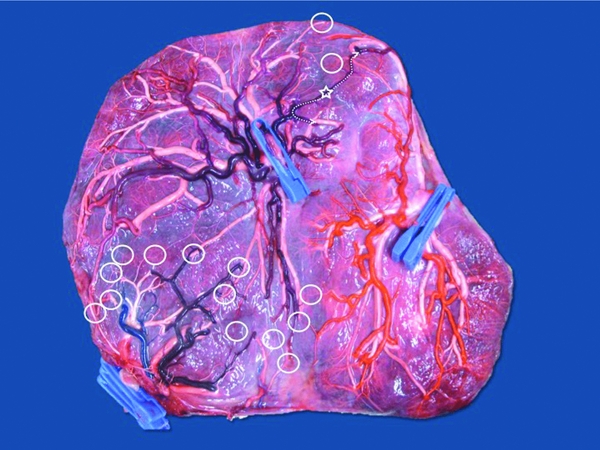
FIGURE 1 Image from a monochorionic triamniotic triplet pregnancy after injection and removal of the fetal membranes. Delivery was at 33 weeks of three healthy neonates, 1850 g, 1675 g, and 1500 g. Triplet 1 has an eccentric cord insertion (one clamp — arteries purple), triplet 2 has a marginal cord insertion (two clamps — arteries red) and triplet 3 has a velamentous insertion (three clamps — arteries blue). There are multiple unidirectional artery-to-vein anastomoses (open circles) between the triplets. There is a bidirectional artery-to-artery anastomosis between triplet 1 and 2 (open Star). Artery-to-artery anastomoses are flexible artery-to-vein anastomoses, as illustrated on the image (dotted line).
As mentioned before, an imbalance in intertwin transfusion through vascular anastomoses is the underlying mechanism of TTTS. We thus expect to find at least one unidirectional AV anastomosis in TTTS placentas. Rarely though, cases of TTTS without AV anastomoses are reported (De Paepe et al., Reference De Paepe, Shapiro, Greco, Luks, Abellar, Luks and Luks2010; van den Wijngaard et al., Reference van den Wijngaard, van Gemert, Lopriore, Vandenbussche, Nikkels and VanBavel2008). As such, arterial stenosis can change the pressure gradient in an AA anastomosis, turning it into a functional AV anastomosis (van den Wijngaard et al., Reference van den Wijngaard, van Gemert, Lopriore, Vandenbussche, Nikkels and VanBavel2008). TTTS thus requires a functional AV connection, rather than a physical one. The total number of AV anastomoses is not increased in TTTS compared to non-TTTS placentas. One study looked at the net cross-sectional area of AV anastomoses, taking into account the number, direction, and diameter of AV anastomoses. Surprisingly, they could demonstrate neither an increased AV imbalance in TTTS placentas, nor a relationship between the direction of AV imbalance and the donor/recipient status (De Paepe et al., Reference De Paepe, Shapiro, Greco, Luks, Abellar, Luks and Luks2010).
AA anastomoses can correct imbalance in intertwin blood flow given their bidirectional nature, and are believed to have a protective effect against TTTS. They have lower resistance than equal size AV anastomoses, and are therefore more efficient in the compensation of imbalanced flow (Umur et al., Reference Umur, Van Gemert, Nikkels and Ross2002). Only 37% of TTTS placentas have an AA anastomosis, in contrast to 87–91% of placentas of uncomplicated monochorionic twins. There is no difference in the size of the AA anastomoses in TTTS and non-TTTS placentas (De Villiers et al., Reference De Villiers, Slaghekke, Middeldorp, Klumper, Walther, Oepkes and Lopriore2012). The protective role of an AA anastomosis in the development of TTTS is supported by the observation that occlusion of an AA anastomosis can lead to acute TTTS (Tan et al., Reference Tan, Denbow, Cox, Talbert and Fisk2004). AA anastomoses can be detected antenatally using Doppler examination, especially in anterior placentas. Pulse wave Doppler examination can detect 75% of AA anastomoses, as they demonstrate a typical bidirectional interference pattern (Fichera et al., Reference Fichera, Mor, Soregaroli and Frusca2005).
VV anastomoses are much rarer in placentas from monochorionic twins not complicated by TTTS, as they are present in only 25% of cases (Lewi et al., Reference Lewi, Cannie, Blickstein, Jani, Huber, Hecher and Deprest2007). They may be more common in TTTS placentas and have been observed in 36% of cases. The difference is even more striking in the subgroup of placentas without AA anastomoses, as the prevalence of VV anastomoses is 32% in these TTTS placentas, compared to only 8% in non-TTTS placentas without AA anastomoses. It is possible that VV anastomoses play a role in the development of TTTS, especially in the absence of AA anastomoses. The venous vessels may be more affected by external pressure due to their low resistance, leading them to act as functional AV anastomoses and thereby contributing to the disease (Zhao et al., Reference Zhao, Cohen, Middeldorp, Klumper, Haak, Oepkes and Lopriore2014). When placentas with and without VV anastomoses are compared, overall fetal demise is higher in the group with VV anastomoses (13% vs. 7%). The presence of VV anastomoses is not an independent risk factor for perinatal mortality, but is rather an independent risk factor for TTTS in the absence of AA anastomoses (de Villiers et al., Reference de Villiers, Zhao, Cohen, van Zwet, Duan, Oepkes and Lopriore2015).
As mentioned before, fetoscopic laser coagulation of placental anastomoses resolves TTTS. The laser procedure has evolved over time. Initially, coagulation was along the intertwin membrane, which caused unnecessary loss of placental territory. Later, only the anastomoses were coagulated selectively, which led to higher double survival rates. However, with this selective technique, anastomoses are missed in up to 21% of cases with double survival. When placentas of cases with double fetal demise were analyzed, missed anastomoses were present in all placentas. Double demise or recurrent TTTS was typically associated with missed large AV anastomoses, without AA or VV anastomoses. When small AV anastomoses were missed, this resulted in discordant hemoglobin levels, now referred to as post-laser TAPS (Lewi et al., Reference Lewi, Jani, Cannie, Robyr, Ville, Hecher and Deprest2006). With the selective technique, recurrent TTTS or post-laser TAPS after primary laser for TTTS are seen in up to 14% and 13% of cases, respectively (Robyr et al., Reference Robyr, Lewi, Salomon, Yamamoto, Bernard, Deprest and Ville2006).
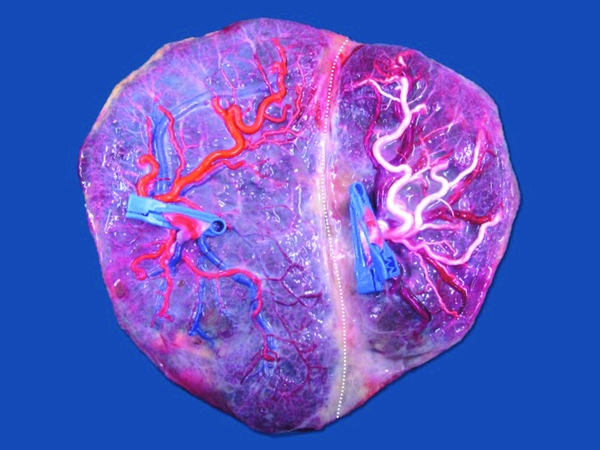
The technique of choice now-a-days is the Solomon technique, and its superiority has been clearly demonstrated in a recent randomized controlled trial. After coagulation of all the visible anastomoses, complete lining of the entire placenta is performed, connecting the coagulation spots from one placental edge to the other (Figure 2). The use of the Solomon technique results in a decreased risk of recurrent TTTS (1% compared to 7% with the standard technique) or post-laser TAPS (3% compared to 16%; Slaghekke et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, Van Zwet, Weingertner and Oepkes2014).
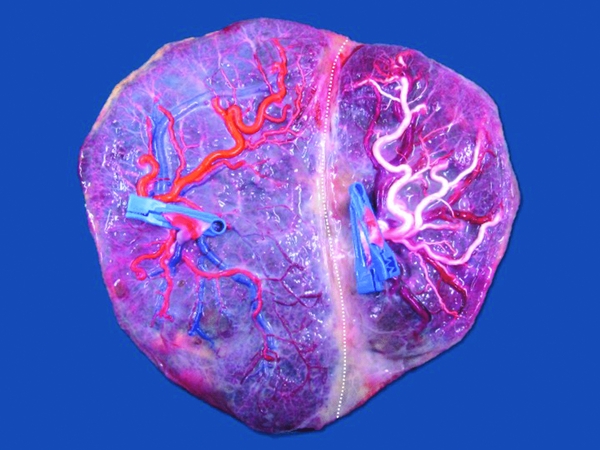
FIGURE 2 Typical placenta after fetoscopic laser coagulation of the vascular anastomoses using the Solomon technique. There is a clear coagulation line (dotted line) that separates the two territories. Fetoscopic laser surgery was performed at 17 weeks. Birth was at 36 weeks of two healthy neonates of 2560 g and 2000 g.
TTTS in monoamniotic twins is rare due to their different placental architecture. The cords are very near to one another, with large anastomoses connecting the two fetal circulations. They also nearly always have an AA anastomosis (Umur et al., Reference Umur, Van Gemert and Nikkels2003). While this might have a protective effect against the development of the more chronic TTTS, these large vessels allow for acute blood shifts and exsanguination, increasing the risk of unpredictable and usually double fetal demise.
Other Placental Features in TTTS
The incidence of velamentous cord insertion is not increased in TTTS compared to non-TTTS cases (Costa-Castro et al., Reference Costa-Castro, De Villiers, Montenegro, Severo, Oepkes, Matias and Lopriore2013; De Paepe et al., Reference De Paepe, DeKoninck and Friedman2005; Lopriore et al., Reference Lopriore, Sueters, Middeldorp, Oepkes, Walther and Vandenbussche2007). However, when TTTS is present, donor twins are more likely to have a velamentous cord insertion compared to recipient twins (24% vs. only 3%; Lopriore et al., Reference Lopriore, Sueters, Middeldorp, Oepkes, Walther and Vandenbussche2007). TTTS placentas more often show an abnormal vascular distribution pattern compared to non-TTTS placentas (60% vs. 44%). Donor twins show an abnormal pattern in 87% of cases, versus only 33% of recipient twins (De Paepe et al., Reference De Paepe, DeKoninck and Friedman2005).
Unequal placental sharing is probably not more common in TTTS, but the donor share is slightly smaller than the recipient share (Lopriore et al., Reference Lopriore, Sueters, Middeldorp, Oepkes, Walther and Vandenbussche2007). When the placenta is unequally shared in TTTS, the smaller part almost always belongs to the donor and when a single umbilical artery is present in a twin pair with TTTS, it is usually the donor twin that is affected (De Paepe et al., Reference De Paepe, Shapiro, Greco, Luks, Abellar, Luks and Luks2010). Unequal sharing in TTTS may increase the risk of fetal demise after laser surgery. At present, we have no means to correctly assess placental sharing antenatally. If we were able to do so, this would help us to better predict the risk of demise.
It is unknown whether the differences in donor and recipient placental characteristics are a cause or the result of TTTS. One hypothesis states that placental perfusion is decreased in the share of the hypovolemic donor, resulting in a reduced expansion of the placental cotyledons on the donor side (Lopriore et al., Reference Lopriore, Sueters, Middeldorp, Oepkes, Walther and Vandenbussche2007). Another hypothesis suggests that TTTS is a condition where angiogenesis is inhibited (Kusanovic et al., Reference Kusanovic, Romero, Espinoza, Nien, Kim, Mittal and Hassan2008).
Magnetic resonance imaging (MRI) in complicated monochorionic pregnancies is a growing field of interest. In a small series of MRI performed in TTTS cases, all TTTS placentas showed an abnormal state of maturation. However, since the same was observed in about two thirds of non-TTTS placentas as well, this finding is unlikely to be clinically useful even if the difference is statistically significant (Linduska et al., Reference Linduska, Messerschmidt, Dekan, Brugger, Weber, Pollak and Prayer2012).
The Placenta in TAPS
In contrast to TTTS that is characterized by a severe discordance in amniotic fluid, TAPS involves a severe discordance in hemoglobin levels. Spontaneous TAPS occurs in 5% of monochorionic twins and typically presents after 26 weeks (Lewi et al., Reference Lewi, Gucciardo, Huber, Jani, Van Mieghem, Doné and Deprest2008). Post-laser TAPS can occur after incomplete laser surgery to correct TTTS, as mentioned before. The incidence is up to 16% in double survivors treated with a selective coagulation of anastomoses, but can be reduced to only 3% with the use of the Solomon technique (Slaghekke et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, Van Zwet, Weingertner and Oepkes2014). Post-laser TAPS usually presents within 1–5 weeks after the initial laser surgery (Robyr et al., Reference Robyr, Lewi, Salomon, Yamamoto, Bernard, Deprest and Ville2006).
TAPS is believed to develop when a chronic net transfusion of red blood cells through very small unidirectional anastomoses takes place. In contrast to TTTS, pregnancies affected by TAPS do not exhibit a severe amniotic fluid discordance and — if present — is by definition less pronounced than in TTTS (Gucciardo et al., Reference Gucciardo, Lewi, Vaast, Debska, De Catte, Van Mieghem and Deprest2010; Slaghekke et al., Reference Slaghekke, Kist, Oepkes, Middeldorp, Klumper, Vandenbussche and Lopriore2009). As in TTTS, laser surgery can be used to coagulate the intertwin anastomoses and thus interrupt the transfusion process and halt the progress of the disease. It may or may not be combined with intra-uterine transfusion, depending on the severity (Lopriore et al., Reference Lopriore, Slaghekke, Oepkes, Middeldorp, Vandenbussche and Walther2010).
TAPS can be diagnosed in the antenatal or postnatal period. During pregnancy, the diagnosis is based on an increased peak-systolic velocity in the middle cerebral artery (MCA-PSV) in the donor twin (>1, 5 multiples of the median) combined with a decrease in MCA-PSV in the recipient twin (<1 multiples of the median). These cut-offs accurately predict anemia and polycythemia in TAPS, as was recently demonstrated (Slaghekke et al., Reference Slaghekke, Pasman, Veujoz, Middeldorp, Lewi, Devlieger and Oepkes2015). After birth, TAPS can be diagnosed based on an intertwin hemoglobin difference of >8 g/dl and one of the two following criteria: either a reticulocyte count ratio of >1.7 or the presence of only small vascular anastomoses (diameter ≤1 mm) on placental inspection (Lopriore et al., Reference Lopriore, Slaghekke, Oepkes, Middeldorp, Vandenbussche and Walther2010). The high reticulocyte count should reflect the chronic nature of TAPS. The small anastomoses allow us to differentiate between TAPS and acute intrapartum transfusion, which is thought to occur suddenly and through large low resistance AA and VV anastomoses (Slaghekke et al., Reference Slaghekke, Kist, Oepkes, Pasman, Middeldorp, Klumper and Lopriore2010).
Anastomoses in TAPS Placentas
TAPS placentas typically have few small and mostly unidirectional AV anastomoses (Lewi et al., Reference Lewi, Gucciardo, Huber, Jani, Van Mieghem, Doné and Deprest2008; Weingertner et al., Reference Weingertner, Kohler, Kohler, Bouffet, Hunsinger, Mager and Favre2010; Zhao et al., Reference Zhao, De Villiers, Slaghekke, Walther, Middeldorp, Oepkes and Lopriore2013). The total number of anastomoses in TAPS placentas is lower compared to normal monochorionic placentas and TTTS cases (Zhao et al., Reference Zhao, De Villiers, Slaghekke, Walther, Middeldorp, Oepkes and Lopriore2013).
AA anastomoses are present in only 14% of cases (19% in spontaneous TAPS and 11% in post-laser TAPS). VV anastomoses are even more rare and were only detected in 7% of post-laser TAPS cases and in none of spontaneous TAPS. The placentas of spontaneous and iatrogenic TAPS cases are thus very similar, although more anastomoses are observed in spontaneous cases (four compared to two). Compensating AV or AA anastomoses are more commonly present in spontaneous TAPS as opposed to post-laser TAPS (75% vs. 37%). In both conditions, anastomoses are more often located towards the edge of the placenta (De Villiers et al., Reference De Villiers, Slaghekke, Middeldorp, Walther, Oepkes and Lopriore2013). AA anastomoses in TAPS are significantly smaller than in uncomplicated monochorionic placentas (0.4 vs. 2.2 mm). These AA anastomoses in TAPS always have a diameter of ≤1 mm, while an AA anastomosis this small is rare in control monochorionic placentas (De Villiers et al., Reference De Villiers, Slaghekke, Middeldorp, Klumper, Walther, Oepkes and Lopriore2012). As mentioned before, AA anastomoses are present in 87–91% of uncomplicated monochorionic twins. Therefore, it is reasonable to assume that the presence of a large AA anastomosis is protective for the development of TAPS.
TAPS is rare in monoamniotic twins. They usually have proximate cord insertions, connected by large intertwin anastomoses (Umur et al., Reference Umur, Van Gemert and Nikkels2003). As TAPS requires only miniscule anastomoses, it seems virtually impossible for this condition to develop in a monoamniotic twin pair. However, one case of TAPS in monoamniotic twins was reported. This case was treated with laser surgery and during fetoscopy, the surgeons could confirm the absence of large anastomoses and the presence of only very thin AV anastomoses (Diehl et al., Reference Diehl, Glosemeyer, Tavares De Sousa, Hollwitz, Ortmeyer and Hecher2013).
Other Placental Features in TAPS
There is no consensus on sharing in spontaneous TAPS, as some authors have reported equally shared placentas and others the opposite (De Villiers et al., Reference De Villiers, Slaghekke, Middeldorp, Klumper, Walther, Oepkes and Lopriore2012; Lewi et al., Reference Lewi, Gucciardo, Huber, Jani, Van Mieghem, Doné and Deprest2008). The largest series does not report a difference in placental sharing between TAPS cases and uncomplicated monochorionic twins. However, they did note that even though 90% of donor twins had a smaller birth weight, 65% of them actually had a larger placental share. In uncomplicated controls, the smaller twin had the smaller placental share in 60% of cases. Birth weight in TAPS seems to be related to the donor versus recipient status, rather than to the placental share (Zhao et al., Reference Zhao, Cohen, Middeldorp, Klumper, Haak, Oepkes and Lopriore2014).
In two studies, an increased incidence of velamentous cords was observed in TAPS placentas, but these were only small series (Lanna et al., Reference Lanna, Consonni, Faiola, Schena, Ratti, Ferrazzi and Rustico2015; Weingertner et al., Reference Weingertner, Kohler, Kohler, Bouffet, Hunsinger, Mager and Favre2010). In a larger series, there was no significant association between velamentous cord insertion and TAPS, on the contrary, there was a tendency towards a lower incidence of velamentous cord insertion in TAPS (Zhao et al., Reference Zhao, Cohen, Middeldorp, Klumper, Haak, Oepkes and Lopriore2014).
A striking feature of TAPS placentas is the color difference on the maternal side, with a very pale placental area belonging to the anemic twin and an extremely dark placental share belonging to the polycythemic twin (Kusanovic et al., Reference Kusanovic, Romero, Gotsch, Mittal, Erez, Kim and Yeo2010; Weingertner et al., Reference Weingertner, Kohler, Kohler, Bouffet, Hunsinger, Mager and Favre2010). In utero, a difference in placental echogenicity can be observed as well, with a hydropic hyperechogenic placental share for the anemic twin, and a relatively hypoechogenic part belonging to the polycythemic twin (Kusanovic et al., Reference Kusanovic, Romero, Gotsch, Mittal, Erez, Kim and Yeo2010; Movva & Rijhsinghani, Reference Movva and Rijhsinghani2014; Slaghekke et al., Reference Slaghekke, Kist, Oepkes, Pasman, Middeldorp, Klumper and Lopriore2010).
Differences and Similarities Between TTTS and TAPS
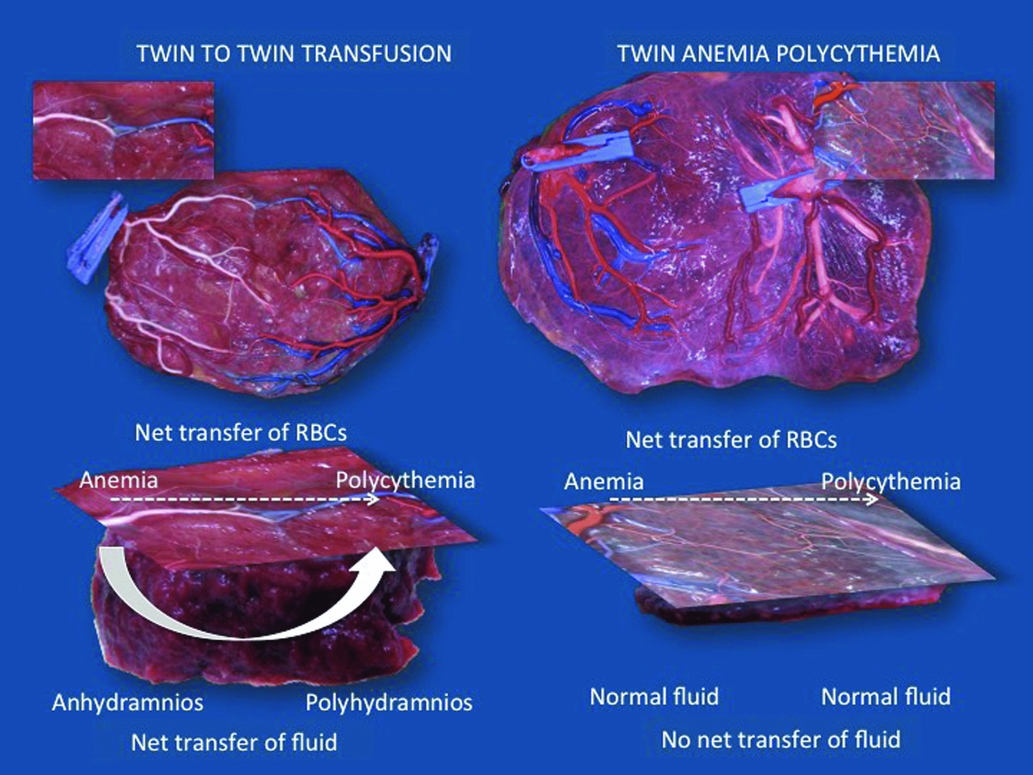
TTTS and TAPS are both transfusion imbalances, but whereas TAPS is characterized by a marked hemoglobin difference, this does not occur in TTTS. As mentioned before, a volume imbalance is an essential part of TTTS. It is unknown why in some cases transfusion is limited to red cells alone and in others it is accompanied by a volume shift. Our hypothesis is that each TTTS initially starts as TAPS, with transfusion of blood from the donor twin to the recipient, resulting in anemia in the donor and polycythemia in the recipient. We would expect fetal anemia to be associated with polyhydramnios. However, in TTTS the donor twin presents with oligohydramnios and the recipient with polyhydramnios. We believe this shift in volume to be a compensatory mechanism to correct for the discordance in hemoglobin. The recipient twin extracts fluid from the donor, thereby masking its polycythemia, while fluid depletion in the donor will mask its anemia. The placenta may play an important role in this fluid exchange process. In TAPS, anastomoses are typically very small. We therefore assume that the shared territory is extremely small in TAPS placentas. In TTTS, however, larger anastomoses are present and the shared part must therefore be larger. This shared territory may explain why fetuses are able to exchange large amounts of fluid in TTTS and not in TAPS (Figure 3). We sometimes see cases of TTTS with discordance in MCA-PSV that fulfills the criteria for TAPS. It is possible that in these cases, compensatory fluid exchange may be insufficient to water down the discordance in fetal hemoglobin.
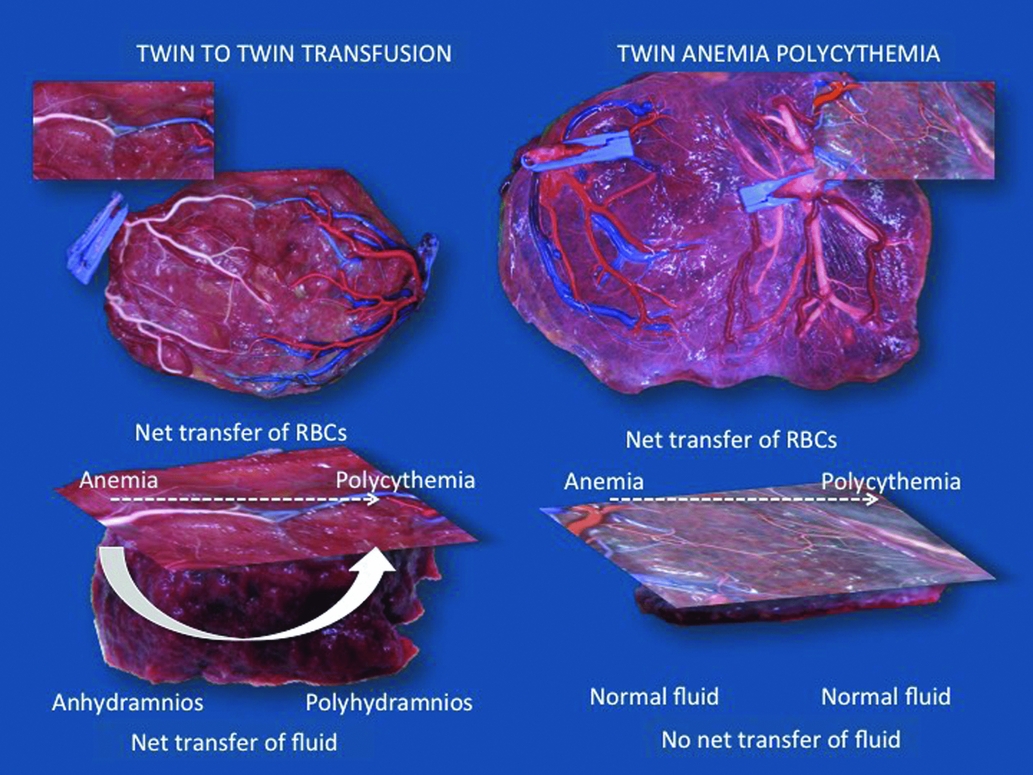
FIGURE 3 Illustration of the difference in size of vascular anastomoses and shared territory between placentas complicated by TTTS and TAPS. The anastomoses in TTTS are considerably larger than the minuscule anastomoses typical of TAPS. Consequently, in TTTS, the shared territory must be larger too. Each TTTS may initially start as a hemoglobin discordance, but through the shared territory, the recipient twin extracts fluid from the donor to mask its polycythemia. On the other hand, fluid depletion in the donor will mask its anemia. In TAPS, the shared territory is extremely small to non-existent, which precludes such compensation. TTTS and TAPS both result from a transfusion imbalance, but whether this results in an amniotic fluid or rather a hemoglobin discordance is determined by the size of the shared territory.
Conclusion
TTTS and TAPS are two distinct pathologies that can only develop in the presence of intertwin anastomoses, but the pathogenesis of both conditions remains not fully understood. Placental examination helps us to gain more insight in the mechanisms behind these transfusion imbalances. This is essential if we want to work towards a prevention of TTTS and TAPS in monochorionic twins.