Introduction
Although the association between diet and breast cancer has been investigated extensively, the overall evidence surrounding the potential relationship between dietary factors and breast cancer carcinogenesis has resulted in the identification of very few risk factors. As with most dietary factors, the association between meat consumption and breast cancer has been equivocal(Reference Linos and Willett1). Some early US and international ecological studies reported positive correlations between rates of breast cancer and per capita intake of meat(Reference Hems2–Reference Gray, Pike and Henderson6), and several factors, such as heterocyclic amines, N-nitroso compounds, polycyclic aromatic hydrocarbons and haem Fe, have been hypothesised as contributing to breast cancer. However, analytical epidemiological studies that assessed red meat and processed meat as dietary intake variables have not corroborated these findings, as associations across cohort and case–control studies have been variable. In a 1993 meta-analysis of seven cohort and case–control studies, Boyd et al. (Reference Boyd, Martin and Noffel7) reported a statistically significant positive association (summary estimate = 1·54; 95 % CI 1·31, 1·82) between red meat intake and breast cancer. In contrast, slight inverse associations for consumption of red meat (summary relative risk (RR) for each 100 g/d increment = 0·98, 95 % CI 0·93, 1·04) or processed meat (summary RR for each 10 g/d increment = 0·98, 95 % CI 0·96, 1·00) were reported in the comprehensive analysis of the Pooling Project of Prospective Studies of Diet and Cancer published in 2002(Reference Missmer, Smith-Warner and Spiegelman8). In a recent meta-analysis among premenopausal women, a non-significant summary association of 1·11 (95 % CI 0·94, 1·31) was reported across three cohort studies, although data from seven cohorts that were analysed in the Pooling Project publication were not included in the analysis(Reference Taylor, Misra and Mukherjee9).
Since these analyses, several large prospective studies have been published that may provide enhanced clarification to any potential associations between red meat consumption and breast cancer. Specifically, evaluations of the NIH-AARP Diet and Health Study(Reference Kabat, Cross and Park10, Reference Cross, Leitzmann and Gail11), Swedish Mammography Cohort(Reference Larsson, Bergkvist and Wolk12), European Prospective Investigation into Cancer and Nutrition (EPIC)(Reference Pala, Krogh and Berrino13), Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial(Reference Ferrucci, Cross and Graubard14), Diet Cancer and Health Cohort(Reference Egeberg, Olsen and Autrup15), Shanghai Breast Self Exam Trial(Reference Shannon, Ray and Wu16), UK Women's Cohort Study(Reference Taylor, Burley and Greenwood17), Monitoring Project on CVD Risk Factors(Reference van der Hel, Peeters and Hein18) and the Nurses' Health Study (I and II)(Reference Cho, Chen and Hunter19–Reference Holmes, Colditz and Hunter22) have been published that provided data on red meat consumption and breast cancer. Therefore, to further update the state of the science, we conducted a review and meta-analysis of prospective cohort studies of red meat or processed meat intake and female breast cancer. We performed high v. low intake meta-analyses, dose–response examinations, heterogeneity assessments, sensitivity and influence evaluations, and an appraisal of publication bias.
Materials and methods
Literature search and study inclusion
We conducted a MEDLINE literature search using the PubMed interface to identify articles eligible for review. All articles indexed by PubMed that were published up to July 2009 were included. The literature search string included: breast cancer OR breast cancers OR breast neoplasm OR breast neoplasms AND (diet* OR diet OR nutrition OR food OR meat OR beef OR pork OR lamb). In addition to the literature search, the bibliographies of review articles pertaining to diet and breast cancer were examined in an effort to identify all available literature that may not have been identified by our database searches. Peer-reviewed publications of prospective cohort studies or nested case–control studies that evaluated red meat or processed meat consumption and female breast cancer were included. Case–control studies, ecological assessments, correlation studies and other publications of aggregate-level analyses were excluded, as were experimental animal studies and mechanistic studies.
Red meat is commonly defined as beef, pork, lamb, or a combination thereof, and processed meat is generally defined as meat made largely from pork, beef or poultry that undergoes methods of preservation, such as curing, smoking or drying(Reference Warriss23, Reference Santarelli, Pierre and Corpet24). Most studies reported associations for categories labelled as ‘red meat’ or ‘processed meat’; however, several studies reported results for individual red (for example, beef, pork) and/or processed (for example, hot dogs, bacon) items. The definitions of red meat and processed meat as a food category varied across studies. While many studies explicitly defined these classifications, other studies reported no description. Studies that reported data for a broad classification of meat, such as ‘total meat’ categories, which included poultry or fish, were excluded. Studies that reported information pertaining to constituents of red meat, such as fat or protein from animal sources, heterocyclic amine exposure, or cooking practices, were obtained but analysis of these factors was beyond the scope of the present assessment. RR and measures of variability (i.e. 95 % CI) for consumption categories of red or processed meat intake using the lowest category of intake as the reference, or available data for such calculations, were required to be reported in the included articles.
Data extraction
Qualitative information and quantitative data were extracted from each study that met the criteria for inclusion. Specifically, information was extracted pertaining to: the year of the study, the study population (i.e. name and nature of the cohort), geographical location of the study, years of follow-up, methods of dietary exposure ascertainment, red meat and processed meat dietary variables and how these variables were defined, the analytical comparison (i.e. the exposure contrast), the number of exposed cases, the RR estimates and 95 % CI, and the factors that were adjusted or controlled for in the analyses.
A thorough review of each article was conducted to identify cohorts that may have been analysed in multiple publications. If results were reported in multiple publications, the inclusion of data was based on (1) the size of the study population, (2) duration of follow-up with an emphasis on the most recent publication with the longest follow-up, (3) classification and analytical categorisation of red or processed meat, and (4) level of control for potential confounding factors. Data from the Missmer et al. (Reference Missmer, Smith-Warner and Spiegelman8) publication were used, which was an analysis of the Pooling Project of Prospective Studies of Diet and Cancer(25), in the primary meta-analysis models. Specifically, Missmer et al. (Reference Missmer, Smith-Warner and Spiegelman8) analysed primary data from eight individual study cohorts from North America and Western Europe, contributing 7379 cases of breast cancer. Data from independent publications of the Nurses' Health Study(Reference Holmes, Colditz and Hunter22, Reference Gertig, Hankinson and Hough26), the Seventh Day Adventist Cohort(Reference Mills, Beeson and Phillips27) and the Netherlands Cohort Study(Reference Voorrips, Brants and Kardinaal28) were not included in the model with Missmer et al. (Reference Missmer, Smith-Warner and Spiegelman8) because these study populations were analysed in the Pooling Project. Data from these studies were included in a separate meta-analysis model that did not include Missmer et al. (Reference Missmer, Smith-Warner and Spiegelman8). Missmer et al. (Reference Missmer, Smith-Warner and Spiegelman8) evaluated data for 1320 breast cancer cases from the Swedish Mammography Cohort during 10 years of follow-up (1987–1997). In a 2009 update of the Swedish Mammography Cohort, Larsson et al. (Reference Larsson, Bergkvist and Wolk12) analysed 2952 breast cancer cases during 20 years of follow-up (1987–2007). Thus, data from Larsson et al. (Reference Larsson, Bergkvist and Wolk12) were included in the primary meta-analyses with Missmer et al. (Reference Missmer, Smith-Warner and Spiegelman8) and data from Larsson et al. (Reference Larsson, Bergkvist and Wolk12) were removed in the sensitivity analyses. Two publications of the Nurses' Health Study I and II cohorts were identified that analysed diet during pre-school(Reference Michels, Rosner and Chumlea21) and adolescence(Reference Frazier, Li and Cho20). Data from these studies were not included in the meta-analysis models because of likely population overlap with other studies. In addition, these studies differed from the other studies included in this assessment in regards to the methodology of past dietary exposure ascertainment and the analysis of diet during early life-time periods. The characteristics of all cohort and nested case–control studies reviewed in the present paper are summarised in Table 1.
Table 1 Summary of cohort studies of red meat and processed meat and female breast cancer
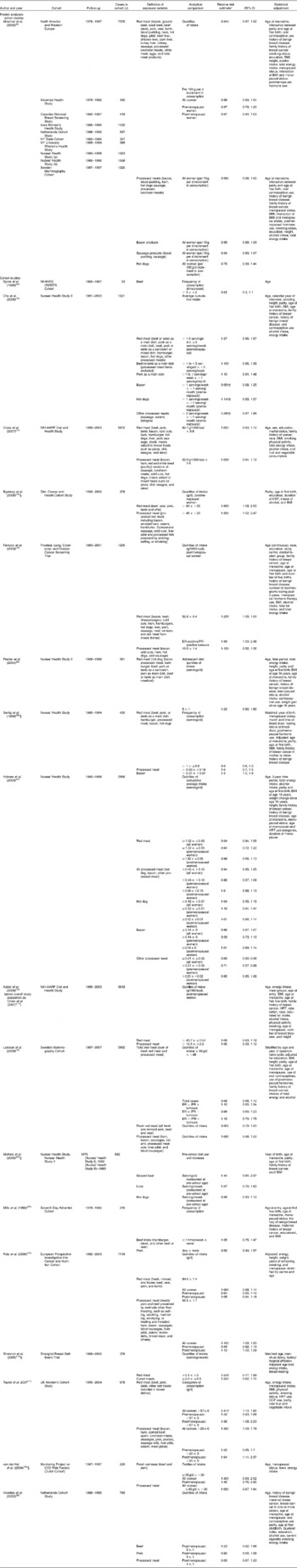
NHANES, National Health and Nutrition Examination Survey; NHEFS, NHANES I Epidemiologic Follow-up Study; HRT, hormone replacement therapy; ER, oestrogen receptor; PR, progesterone receptor; OCP, oral contraceptive pill.
* Highest v. lowest intake comparison unless otherwise noted.
‡ Risk estimates combined using fixed-effects model before inclusion in primary meta-analysis.
§ Nested case–control study.
Statistical analyses
Meta-analyses comparing the highest intake category of red meat or processed meat with the lowest (or referent) intake category were conducted. Meta-analysis models were constructed for overall red meat or processed meat groups as well as for individual meat items where applicable (for example, hot dogs, bacon, organ products). In two studies(Reference Larsson, Bergkvist and Wolk12, Reference Cho, Chen and Hunter19) that reported data for total red meat (including processed meat items) and red meat only, data were selected specifically for red meat (without processed meat items). Separate meta-analyses were generated among the studies that reported data by menopausal status. Additionally, meta-analyses of dose–response categorical data were conducted using the method proposed by Greenland & Longnecker(Reference Greenland and Longnecker29), in which the linear dose–response slope is calculated for each study while accounting for the correlation across intake categories within a study(Reference Berlin, Longnecker and Greenland30). If the number of cases and person-time data were not available for each intake strata, variance-weighted least squares regression was utilised to estimate the slope coefficient. Different intake units were reported across studies; therefore, we used 80 g as the approximate serving size for red meat and 30 g for processed meat.
Fixed-effects and random-effects models were used to calculate summary RR estimates (SRRE), 95 % CI, and corresponding P values for heterogeneity. In the ‘one study removed’ sensitivity analyses, the relative influence of each study on the model-specific SRRE was examined by generating an SRRE based on all studies in a particular model, followed by the removal of one study at a time in order to compare the overall SRRE with SRRE from models that had one study removed. The presence of publication bias was assessed visually by examining a funnel plot measuring the standard error as a function of effect size, as well as performing Duval and Tweedie's trim and fill method(Reference Rothstein, Sutton and Borenstein31). All statistical analyses were performed using STATA (version 10.0; StataCorp LP, College Station, TX, USA), Comprehensive Meta-Analysis (version 2.2.046; Biostat, Englewood, NJ, USA) and Episheet(Reference Rothman32). The utilisation of independent analytical programs allowed for the validation of calculations.
Results
Red meat
No significant association between the highest category of red meat intake compared with the lowest category of intake and breast cancer was observed in the meta-analysis model that included data from the Pooling Project publication (eight cohorts) combined with data from ten additional studies (SRRE for fixed-effects model = 1·02; 95 % CI 0·98, 1·07; P value for heterogeneity = 0·001) (Fig. 1; Table 2). The SRRE for the random-effects model was slightly stronger in magnitude (SRRE 1·07; 95 % CI 0·98, 1·17), primarily because this model provided only 16 % of the relative weight to the pooled analysis of eight cohorts by Missmer et al. (Reference Missmer, Smith-Warner and Spiegelman8). Byrne et al. (Reference Byrne, Ursin and Ziegler33) reported data only for beef consumption; therefore, this study was removed as part of the sensitivity analysis. This study had less than 1 % of relative weight, so the overall summary estimate remained virtually unchanged with its removal. In the one study removed influence analysis, the removal of any single study did not appreciably alter the overall SRRE by more than 4 %. When Larsson et al. (Reference Larsson, Bergkvist and Wolk12) was removed (partial overlap with Missmer et al. (Reference Missmer, Smith-Warner and Spiegelman8)), the fixed- and random-effects summary associations became 1·04 (95 % CI 0·99, 1·08; P value for heterogeneity = 0·002) and 1·10 (95 % CI 1·00, 1·21), respectively. Replacing the data from Missmer et al. (Reference Missmer, Smith-Warner and Spiegelman8) with data from studies that analysed populations included in the Pooling Project (i.e. Holmes et al. (Reference Holmes, Colditz and Hunter22), Mills et al. (Reference Mills, Beeson and Phillips27) and Voorrips et al. (Reference Voorrips, Brants and Kardinaal28)) did not markedly modify the overall summary associations (SRRE for fixed-effects model = 1·03; 95 % CI 0·99, 1·08; P value for heterogeneity = 0·005; SRRE for random-effects model = 1·06; 95 % CI 0·98, 1·15) nor did this model explain the observed heterogeneity. The summary associations in a sensitivity analysis that included only studies(Reference Cross, Leitzmann and Gail11–Reference Cho, Chen and Hunter19, Reference Holmes, Colditz and Hunter22) published after the Pooling Project publication were similar in magnitude to the overall association (fixed-effects SRRE 1·05; 95 % CI 1·00, 1·10; P value for heterogeneity = 0·023; random-effects SRRE 1·08; 95 % CI 1·00, 1·17) (Table 2).
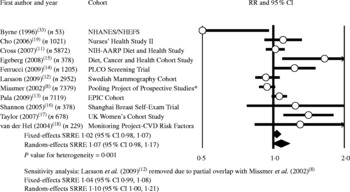
Fig. 1 Meta-analysis of prospective studies of red meat and breast cancer. * Includes data pooled from eight cohorts; partial overlap with Larsson et al. (2009)(Reference Larsson, Bergkvist and Wolk12). RR, relative risk; NHANES, National Health and Nutrition Examination Survey; NHEFS, NHANES I Epidemiologic Follow-up Study; PLCO, Prostate, Lung, Colorectal, and Ovarian; EPIC, European Prospective Investigation into Cancer and Nutrition; SRRE, summary relative risk estimate.
Table 2 Summary of meta-analysis results of red meat and processed meat and breast cancer
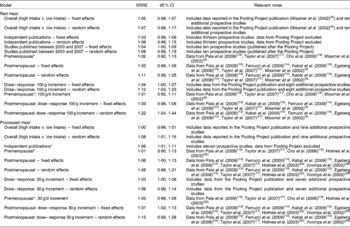
SSRE, summary relative risk estimate.
* Fixed-effects model; results similar for random effects.
The SRRE for the studies that reported data for red meat and breast cancer among premenopausal women was 1·02 (95 % CI 0·92, 1·13; P value for heterogeneity = 0·268; fixed-effects model) (Table 2). The fixed-effects SRRE among studies that reported red meat intake data for postmenopausal women was 1·02 (95 % CI 0·98, 1·08; P value for heterogeneity = 0·005), while the summary association in the random-effects model was slightly stronger (SRRE 1·11; 95 % CI 0·99, 1·25), largely due to the reduction of relative weight given to the pooled analysis by Missmer et al. (Reference Missmer, Smith-Warner and Spiegelman8).
In the categorical dose–response meta-analysis, the SRRE for each 100 g increment of red meat intake was 1·04 (95 % CI 1·00, 1·07; P value for heterogeneity < 0·0001) in the fixed-effects model and 1·12 (95 % CI 1·03, 1·23) in the random-effects model. Among premenopausal women, the summary association for each 100 g increment of red meat was 1·01 (95 % CI 0·92, 1·11; fixed effects) with a non-significant P value for heterogeneity (P = 0·316). Among postmenopausal women, the fixed-effects and random-effects SRRE for each 100 g increment of red meat intake were 1·03 (95 % CI 0·98, 1·08; P value for heterogeneity < 0·0001) and 1·22 (95 % CI 1·04, 1·44), respectively. Modest differences in summary associations by model were observed among postmenopausal women, largely due to the fact that the random-effects model provided only 22 % of the total weight to the Pooling Project data from Missmer et al. (Reference Missmer, Smith-Warner and Spiegelman8) (which included eight cohorts) and 78 % of the relative weight to the five additional studies in this model. The fixed-effects model more appropriately provided 58 % of the relative weight to Missmer et al. (Reference Missmer, Smith-Warner and Spiegelman8).
Processed meat
No association was observed in the fixed-effects meta-analysis of processed meat intake and breast cancer (SRRE 1·00; 95 % CI 0·98, 1·01; P value for heterogeneity = 0·005) (Table 2; Fig. 2). This model provided 85 % of the relative weight to data from the Pooling Project analysis (seven cohorts). In contrast, the random-effects model provided only 23 % of relative weight to this study, resulting in a slightly greater summary association (SRRE 1·08; 95 % CI 1·01, 1·16). The Pooling Project data represented a 10 % increment of processed meat intake rather than a ‘high’ intake quantile. Removal of data from this study, and inclusion of data from individual publications of the Netherlands Cohort study(Reference Voorrips, Brants and Kardinaal28) and the Nurses' Health Study(Reference Holmes, Colditz and Hunter22), both of which analysed study populations included in the publication by Missmer et al. (Reference Missmer, Smith-Warner and Spiegelman8), resulted in summary associations of 1·06 (95 % CI 1·01, 1·11) and 1·07 (95 % CI 1·01, 1·14) for fixed- and random-effects models, respectively. Meta-analysis of four studies that reported data for premenopausal women resulted in an SRRE of 1·01 (95 % CI 0·90, 1·13) (same result for fixed and random effects) (Table 2). The summary association was slightly greater among the seven studies that reported data for postmenopausal women (fixed-effects SRRE 1·06; 95 % CI 1·00, 1·13; P value for heterogeneity = 0·051; random-effects SRRE 1·09; 95 % CI 0·99, 1·21) (Table 2).
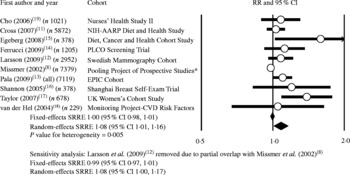
Fig. 2 Meta-analysis of prospective studies of processed meat and breast cancer. * Includes data pooled from seven cohorts; partial overlap with Larsson et al. (2009)(Reference Larsson, Bergkvist and Wolk12). RR, relative risk; PLCO, Prostate, Lung, Colorectal, and Ovarian; EPIC, European Prospective Investigation into Cancer and Nutrition; SRRE, summary relative risk estimate.
The SRRE for each 30 g increment of processed meat intake was 1·03 (95 % CI 1·00, 1·06; P value for heterogeneity < 0·0001) in the fixed-effects model and 1·06 (95 % CI 0·99, 1·14) in the random-effects model. Among premenopausal women, the summary association for each 30 g increment of processed meat was 1·03 (95 % CI 0·98, 1·08; fixed effects) with a non-significant P value for heterogeneity (P = 0·535). Among postmenopausal women, the fixed-effects and random-effects SRRE for each 30 g increment of processed meat intake were 1·07 (95 % CI 1·02, 1·13; P value for heterogeneity < 0·0001) and 1·13 (95 % CI 0·99, 1·28), respectively.
Two studies(Reference Cho, Chen and Hunter19, Reference Holmes, Colditz and Hunter22) of the Nurses' Health Study I and II cohorts reported categorical intake data for hot dogs, bacon and other processed meat (sausage, salami, bologna), and one study(Reference Ferrucci, Cross and Graubard14) reported data for bacon and sausage. Meta-analysis of the highest v. lowest intake of bacon resulted in an SRRE of 1·01 (95 % CI 0·92, 1·12; P value for heterogeneity = 0·752), and the SRRE for hot dogs was 1·05 (95 % CI 0·96, 1·15; P value for heterogeneity = 0·589) (data not shown). In the meta-analysis of intake of other processed meat, the SRRE was 1·16 (95 % CI 0·98, 1·39). Similarly, non-significant associations were reported for each 10 g/d increment of bacon (pooled RR 0·99; 95 % CI 0·89, 1·09) and sausage (pooled RR 0·94; 95 % CI 0·83, 1·07) in the Pooling Project(Reference Missmer, Smith-Warner and Spiegelman8) analysis of seven cohorts. In addition, an inverse association for each 100 g/d increment of hot dogs was observed (pooled RR 0·75; 95 % CI 0·39, 1·44)(Reference Missmer, Smith-Warner and Spiegelman8).
Publication bias
In the assessment of prospective studies of red meat intake and breast cancer, the point estimates were skewed slightly to the right of the weighted effect size, indicating potential publication bias (Fig. 3). Using Duval and Tweedie's trim and fill method, in which the summary association is recomputed based on the imputation of potentially missing studies, resulted in changing the SRRE from 1·02 to 1·01 and from 1·07 to 1·01 for the fixed- and random-effects models, respectively (based on imputing three studies). Six studies were imputed for the processed meat analysis, resulting in changing the SRRE from 1·08 (95 % CI 1·01, 1·16) to 1·00 (95 % CI 0·94, 1·06) based on the random-effects model (note: the SRRE was virtually unchanged for the fixed-effects model).
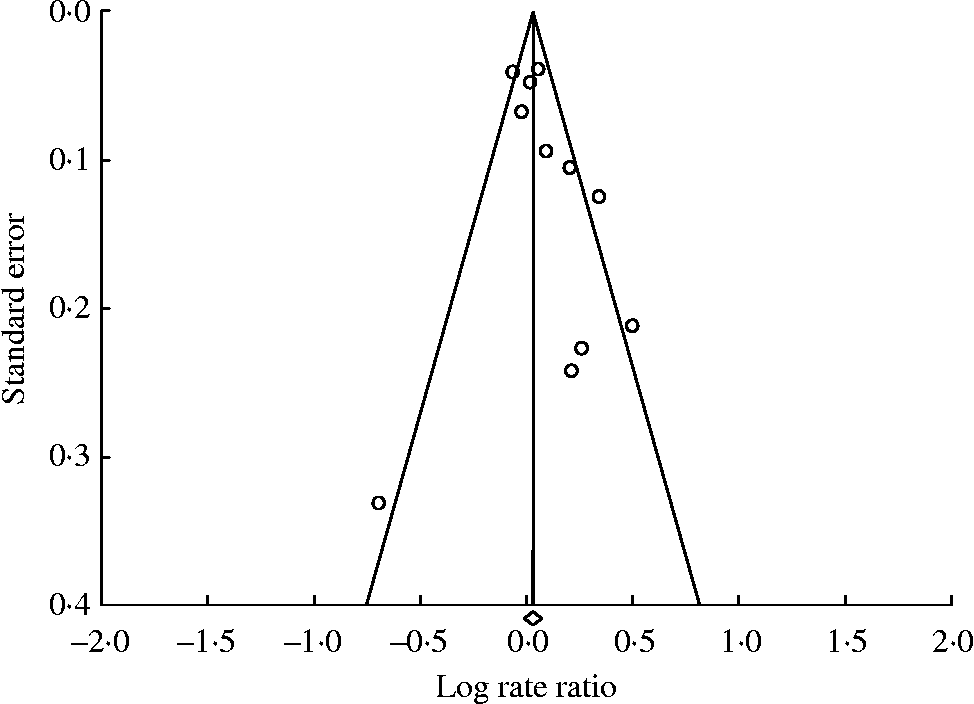
Fig. 3 Funnel plot for prospective studies of red meat and breast cancer. For studies, see Fig. 1.
Discussion
Since the publication of the Pooling Project analysis of eight cohorts in 2002(Reference Missmer, Smith-Warner and Spiegelman8), several large prospective studies have been published that evaluated the relationship between red meat and processed meat consumption and breast cancer. Therefore, the objectives of our quantitative assessment were to synthesise and summarise data across all available prospective studies to update the state of the science, to better clarify any associations, and to identify potential sources of heterogeneity. Overall, most associations across the variety of meta-analysis models were slightly above the null value (i.e. 1·0) and not statistically significant. Significant heterogeneity was evident in most meta-analysis models, and the heterogeneity did not appear to be explained by menopausal status or by year of publication. Moreover, adjusting for publication bias resulted in attenuating summary associations.
In this quantitative assessment, data for red meat intake and breast cancer from ten prospective studies were combined with pooled data reported in the Pooling Project publication(Reference Missmer, Smith-Warner and Spiegelman8). Thus, we were able to meta-analyse data on over 25 000 cases of breast cancer. Among all women, no statistically significant associations were observed in the high v. low red meat intake analyses, with SRRE of 1·02 and 1·07 for the fixed- and random-effects models, respectively. Although these summary associations were not indicative of a significant increased risk of breast cancer among consumers of red meat, significant heterogeneity was observed between the effect estimates in this analysis. The heterogeneity did not seem to be explained by selection of cohort data, as removal of data from the Pooling Project study did not modify the summary associations nor did analysing data from studies published after the Pooling Project. Moreover, fixed-effects summary associations were identical (i.e. 1·02) in the analyses of premenopausal women and postmenopausal women. Therefore, the heterogeneity in effect sizes is not probably due to variability in associations by menopausal status.
High v. low intake meta-analysis only takes into account the highest level of intake (compared with the referent group) as reported in a particular study, and the data for the middle intake categories are not typically analysed. In contrast, categorical dose–response regression meta-analysis utilises all available data for each intake strata to produce a summary estimate reflecting risk per incremental level of intake. Although this type of analysis uses all available data, it is assumed that risk increases (or decreases) linearly, and risk may be extrapolated to intake levels considerably higher than what is reported in an individual study. Indeed, we used 100 g increments in the meta-analyses of red meat intake to be consistent with the level reported in the Pooling Project analysis even though this intake level is generally higher than the reported intakes in the majority of cohorts. Similar to the high v. low intake analyses, summary associations for each 100 g increment of red meat intake were weakly elevated, but significant heterogeneity was observed (Table 2). Dose–response summary associations by menopausal status were similar for the fixed-effects meta-analyses, but the random-effects summary association was modestly stronger (SRRE = 1·22) among postmenopausal women. This difference was due to less relative weight given to the Pooling Project data of eight cohorts and more weight given to the five other studies. In the Pooling Project analysis, the pooled RR for each 100 g increment of red meat across eight cohorts was 0·97. In contrast, the summary association across five studies of postmenopausal women published after the Pooling Project was 1·13 and 1·34 for the fixed- and random-effects models, respectively, although significant heterogeneity remained (sensitivity analysis data not shown).
Meta-analyses of processed meat intake and breast cancer were similar to the analyses of red meat. Summary associations ranged between 1·00 and 1·13 and most were not statistically significant. Among all women, no association was observed in the fixed-effects analysis of high v. low intake, while a summary association of 1·08 was observed in the random-effects model. This difference was largely due to less relative weight provided to the Pooling Project data. When data from the Pooling Project were excluded, the fixed- (i.e. 1·06) and random- (i.e. 1·07) effects models produced similar summary associations, although significant heterogeneity was observed. Summary associations were slightly stronger, albeit weakly elevated, in the analyses of postmenopausal women compared with premenopausal women; however, the CI were largely overlapped. Although data were relatively sparse, analyses of individual processed meat items (for example, bacon, hot dogs) were not supportive of significant associations with breast cancer.
Statistically, based on the available epidemiological data, we were unable to identify significant sources of heterogeneity, although between-study variability was present in most meta-analysis models. From a methodological standpoint, regardless of statistical heterogeneity, variability can be produced by a wide array of factors, such as the type of study population, the methods of dietary assessment and/or measurement, variable definitions (for example, food groups, serving sizes), analytical categorisations (for example, servings per week, g per d), exposure contrasts (analytical cut-points and comparisons of intake levels), and degree of adjustment for potential confounding factors. Despite these sources of potential variability, collectively, the meta-analyses produced relatively consistent results, with most summary associations just above the null value.
Although we were able to conduct a wide variety of meta-analyses for red/processed meat intake and breast cancer, data are relatively sparse for some emerging hypotheses. Indeed, how and/or whether diet early in life may contribute to the development of adult cancer is of increasing scientific interest. Of particular relevance is breast cancer because of increased mammary susceptibility to potential carcinogens during adolescence and early life(Reference Linos and Willett34). In a case–control study nested within the Nurses' Health Study I and II cohorts, Michels et al. (Reference Michels, Rosner and Chumlea21) examined the potential effects of pre-school diet on subsequent breast cancer risk later in life. The mothers of participants in the Nurses' Health Study cohorts were asked about their daughters' perinatal and early childhood dietary habits using a thirty-food item questionnaire. Ground beef was associated with a non-significant 44 % increased risk of breast cancer, while consumption of meat (as a main dish or as a sandwich or mixed dish) or hot dogs were associated inversely, albeit non-significantly, with subsequent breast cancer risk. In a study of the Nurses' Health Study II cohort(Reference Frazier, Li and Cho20), participants were asked to complete a questionnaire regarding diet during high school, a life period that may be affected by micronutrient intake during adolescent growth. A non-significant positive association between the highest intake of red meat and subsequent risk of breast cancer was reported (RR 1·22; 95 % CI 0·82, 1·82), but no trend based on incremental intake was observed (P value for trend = 0·17). Interpretation of these studies should be made with some reservation, as results may be subject to poor recall since study participants (or mothers of cases) are reporting dietary habits that probably occurred 30–40 or more years before ascertainment. Although an intriguing area of research, no conclusions at the present time can be drawn regarding the possible association between pre-school or adolescent meat consumption and adult breast cancer risk because of limited data.
Another area of increasing scientific interest is the potential relationship between dietary factors and breast cancer risk according to tumour hormone receptor status. Although breast tumours differ clinically and biologically by hormone receptor status(Reference Cho, Chen and Hunter19), there is little evidence regarding the potential association between red/processed meat and hormone receptor status cancer. In an analysis of the Nurses' Health Study II, Cho et al. (Reference Cho, Chen and Hunter19) evaluated whether the association between red meat or processed meat and breast cancer differed by hormone receptor status among premenopausal women. As reported earlier, the authors found a non-significant positive association between the highest intake quintile of red meat and total breast cancer (RR 1·27; 95 % CI 0·96, 1·67). The positive association, however, was restricted to women with hormone receptor-positive cancer (oestrogen receptor (ER)+/progesterone receptor (PR)+) (RR 1·97; 95 % CI 1·35, 2·88). A non-significant inverse association was reported among women with hormone receptor-negative cancer (ER − /PR − ) (RR 0·89; 95 % CI 0·43, 1·84). Positive associations were also reported for pork (as a main dish), hamburger, bacon, hot dogs, and other processed meats (for example, sausage, salami, bologna) among women with ER+/PR+ cancer, while inverse associations for these same meat groups were observed among women with ER − /PR − cancer. In contrast, in a recent analysis of the Swedish Mammography Cohort(Reference Larsson, Bergkvist and Wolk12), stronger associations for red meat intake were found among women with ER − /PR − cancer (RR 1·12; 95 % CI 0·70, 1·79) than ER+/PR+ cancer (RR 1·10; 95 % CI 0·90, 1·34). An inverse association for the highest red meat intake category was reported among women with ER+/PR − cancer (RR 0·86; 95 % CI 0·60, 1·23).
It has been hypothesised that mutagenic by-products, such as heterocyclic amines or polycyclic aromatic hydrocarbons, of cooking meat may contribute to mammary carcinogenesis. However, findings from epidemiological studies of heterocyclic amines and polycyclic aromatic hydrocarbons and breast cancer have been variable and limited to few investigations(Reference Kabat, Cross and Park10, Reference Ferrucci, Cross and Graubard14, Reference De Stefani, Ronco and Mendilaharsu35–Reference Delfino, Sinha and Smith38). Some studies(Reference Ferrucci, Cross and Graubard14, Reference Deitz, Zheng and Leff39–Reference Zheng, Gustafson and Sinha41) have shown that consumption of well-done meat is associated positively with increasing the risk of breast cancer, but other studies(Reference Kabat, Cross and Park10, Reference Gertig, Hankinson and Hough26, Reference Delfino, Sinha and Smith38) have found no such effect. In a recent prospective analysis of 3818 postmenopausal breast cancer cases in the NIH-AARP Diet and Health Study cohort, no associations were found for meat cooked at high temperatures, well/very well-done cooked meat, overall mutagenic activity, or specific heterocyclic amines, and the authors concluded that their analysis ‘provides no support for a role of meat mutagens in the development of postmenopausal breast cancer’(Reference Kabat, Cross and Park10). In another recent analysis of postmenopausal women(Reference Ferrucci, Cross and Graubard14), no significant associations were reported for well/very well-done cooked meat or overall mutagenic activity, but a significant positive association was found for 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx). No linear trend was observed for MeIQx, however.
The relationship between meat consumption and breast cancer has been the focus of several epidemiological investigations, yet there has been no clear scientific consensus as to whether red or processed meat intake increases the risk of breast cancer. The current quantitative assessment summarises prospective data on over 25 000 cases of breast cancer, and incorporates data from several recently published cohorts. The results of this meta-analysis do not appear to support an independent association between red meat or processed meat intake and breast cancer. Collectively, all summary associations were weakly elevated, with most ranging between 1·00 and 1·10. Some analyses produced statistically significant associations, although results were sensitive to the choice of model (fixed effects v. random effects). Heterogeneity was evident in most meta-analysis models, and this between-study variability could not be explained by analyses of menopausal status, year of publication, or inclusion/exclusion of specific cohorts. In addition, there was modest evidence of publication bias which may have skewed the summary associations slightly in the positive direction. Breast cancer is a heterogeneous disease with differing aetiologies; thus, the potential role that diet may play in the development of breast cancer among subgroups is of great public health importance. Recent studies have suggested that meat consumption may affect breast cancer risk through hormone receptor status, and that diet early in life may influence adult breast cancer. Data for these hypotheses are limited, however, and additional prospective studies are needed before conclusions can be drawn.
Acknowledgements
The present review received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The present study was partially supported by the Cattlemen's Beef Board, through the National Cattlemen's Beef Association (NCBA); and the National Pork Board. NCBA and the National Pork Board did not contribute to the writing, analysis, or interpretation of the research findings. All data included in the present paper were extracted from peer-reviewed published literature. All analyses performed are transparent and reproducible.
D. D. A. contributed to the writing, analysis, and literature review; L. M. M. contributed to the data extraction and literature review; P. J. M. contributed to the writing, technical and editorial review; C. A. C. contributed to the data extraction, database management, and editorial review.
The authors declare no conflicts of interest.









