INTRODUCTION
Herpes zoster (HZ) results from the reactivation of the latent varicella-zoster virus (VZV) acquired following primary varicella infection (chickenpox). HZ is characterized by a unilateral dermatomal rash and pain, which usually lasts between 2 weeks and 1 month [Reference Oster1–Reference Jung3]. The most common complication is post-herpetic neuralgia (PHN), which is often severe and significantly impairs quality of life [Reference Schmader4]. There is still no international consensus on the definition of PHN even if a recent trend seems to only consider pain as PHN when persisting more than 90 days after rash onset. Therefore, we proposed to explore two PHN definitions in this project: pain occurring or persisting at least 1 or 3 months after rash onset.
The HZ lifetime risk has been reported to be 23% [Reference Miller, Marshall and Vurdien5] to 30% [Reference Brisson6] while recurrent zoster is rare, with a reported lifetime risk of 1–5% [Reference Cunningham and Dworkin7]. The risk of developing HZ increases considerably with age, roughly doubling every decade after the age of 50 [Reference Donahue8]. After the age of 50 years, about 20% of patients with HZ will develop PHN [Reference Brisson6, Reference Bowsher9, Reference Edmunds, Brisson and Rose10] and this complication occurs more often in older patients. Other reported risk factors for the development of PHN are female gender, the length of prodromal pain and rash severity, greater degree of sensory impairment, ophthalmic location and psychological distress [Reference Jung3, Reference Schmader4, Reference Opstelten11].
Although HZ and PHN incidence is thought to be high, data on epidemiology and costs related to HZ and PHN in the United Kingdom are scarce. To our knowledge, only one observational study, published by Scott et al. [Reference Scott12] has described HZ and PHN incidence in the United Kingdom since 1975 [Reference Hope-Simpson13]. This study also provided information on costs on a limited sample (n=96).
Recent research has demonstrated that a VZV vaccine could substantially reduce the incidence of HZ and resulting complications [Reference Oxman14]. In order to understand the potential impact of introducing the vaccine in the United Kingdom, data related to the epidemiology and burden of disease are required.
The present study aimed to update existing epidemiological data related to HZ and its most common complication, PHN, to understand the clinical management of the diseases in primary care and to estimate the associated costs of management in the United Kingdom from the National Health Service (NHS) perspective.
METHODS
The epidemiological and cost data were estimated in immunocompetent individuals aged ⩾50 years, using a large primary-care dataset and official hospital episode statistics.
Data source
Data were extracted from the UK General Practice Research Database (GPRD), which contains about 35 million patient-years of data dating back to 1987 and 3 million active patient records from 603 UK GP practices. Data include demographic information, lifestyle factors, medical diagnosis and symptoms, prescription drugs, referrals to hospitals or specialists entered by the GP, treatment outcomes and patient registration, practice and consultation details.
As the GPRD is supplied by GPs, non-elective hospitalizations are rarely reported. Number of hospitalizations and mean length of stay were supplemented by Hospital Episode Statistics (HES [15]), which is a national statistical data warehouse of the hospital attendances in England. Data are collected from all NHS trusts and relate to clinical information (diagnosis, type of procedure, hospital length of stay), administration (date of admission, time waited), patient's demographics (age, gender, ethnicity) and geographical details of the patients' residence and place of care.
HZ incidence
The analysis involved a retrospective evaluation of health-care records of immunocompetent patients aged ⩾50 years, with a diagnosis, identified by recorded codes (listed in the Appendix, available online), of HZ occurring between 1 January 2000 and 31 March 2006. Patients were observed from their first HZ diagnosis until the end of follow-up in the GPRD (due to death or transfer to another GP practice). Patients with at least 1 year of follow-up before and after HZ diagnosis were included. Incidence of HZ was obtained by dividing the number of incident cases during the study period by the person-time contribution of the population at risk, which included all immunocompetent individuals aged ⩾50 years who had at least 2 years of follow-up in the GPRD. Immunocompromised individuals were identified as patients who had received a transplant or with HIV and were excluded from the study population. For patients experiencing subsequent episodes of HZ over the study period, only the first episode was included as an incident case as this study focused on non-recurrent zoster.
Proportion of patients developing PHN
PHN may be defined as pain occurring or persisting for at least 1 month or 3 months after rash onset. These two definitions were explored in the analysis. As not all cases of PHN are coded as such in general practice, episodes were identified via recorded diagnosis of PHN and prescription of specific neuropathic pain medications in patients with a previous diagnosis of HZ. Medications included topical analgesics, tricyclic antidepressants (TCAs), anticonvulsants and tramadol. To minimize misidentification of PHN, patients with conditions requiring the same type of medications (diabetic neuropathy, epilepsy and recurrent HZ) were excluded from the study.
Patients diagnosed with PHN via a recorded code or prescribed typical neuropathic pain medication at least 1 month and <12 months after HZ diagnosis were identified as patients developing PHN using the 1-month definition.
Similarly, patients diagnosed with PHN via a recorded code or prescribed typical neuropathic pain medications at least 3 months and <12 months after HZ diagnosis were denoted as PHN patients using the 3-month definition.
Duration, severity and health-care resource use
HZ
As disease duration and severity are rarely reported in the GPRD, acute HZ was assumed to last no longer than 1 month [Reference Oxman14] and the severity of pain was assessed via the strongest analgesics prescribed within the month of the HZ diagnosis (no analgesic: no pain; non-opioid analgesics: mild pain; weak opioid analgesics: moderate pain; strong opioid analgesics or co-codamol 30/500 or tramadol: severe pain).
Typical health-care resource use occurring within the month of the acute HZ was assumed to relate to HZ, and analysed by type, i.e. GP visits, secondary-care visits and medications.
PHN
The earliest date between the PHN diagnosis and the first prescription of specific neuropathic pain medication (at least 1 or 3 months after the HZ index date according to the definition used) defined the PHN index date. The sequence of prescriptions was used to estimate the end date of the PHN episode. Resulting PHN duration was explored over the whole study population and by level of severity.
Similar to that of HZ, severity of PHN was assessed on the basis of prescribed medications. For most patients, the pain lessens over time and therefore, prescriptions occurring within the month of the PHN index date were used as a proxy to assess the severity of PHN at diagnosis. Patients prescribed non-opioid analgesics, TCAs, anticonvulsants or topical analgesics were identified as mild; patients switching from TCAs to anticonvulsants or vice versa, or adding a treatment to an existing treatment (except opioid analgesic and co-codamol 30/500) were identified as moderate and patients prescribed opioid analgesics or adding a weak opioid analgesic after a switch in therapy or prescribed co-codamol 30/500 were identified as severe patients.
For each cohort of PHN patients (using the 1-month and 3-month definitions), typical health-care resource use occurring between the PHN index date and the PHN end dates were assumed to relate to PHN and analysed by type, i.e. GP visits, secondary-care visits and medications.
Economic analysis
Mean cost of HZ and PHN outpatient management
Costs of medications and outpatient visits were obtained by individually costing the resource use items per patient, applying standard 2006 unit costs [Reference Curtis and Netten16, 17] (see online Appendix). Where needed, costs were inflated to 2006 levels using the consumer price index. A monthly cost of PHN was obtained by dividing the total cost over the PHN episode by its duration.
Mean hospital costs
The number of admissions due to HZ and PHN in England and mean length of hospital stay were taken from HES 2004–2005 using ICD-10 codes (HZ: B02 codes excluding B02.2 and PHN: B02.2). The daily cost associated with a non-elective in-patient stay due to viral illness from the NHS Reference Costs [18] (£117.00) was applied to calculate the total hospitalization cost per year due to HZ and PHN in England.
In order to estimate the mean hospital cost per patient, the numbers of HZ and PHN hospitalized cases occurring in England were assessed, by applying the HZ and PHN incidence rate estimates from the GPRD to the population size [19]. The 1-month definition of PHN was used to estimate the number of PHN episodes in order not to underestimate the number of cases (which would lead to an overestimation to the cost per episode).
The mean hospital cost per HZ case was obtained by dividing the total cost of hospitalizations due to HZ by the estimated number of HZ cases. The same approach was used to estimate the mean cost of hospitalization per PHN episode.
RESULTS
Epidemiology
HZ incidence
A total of 27 225 incident cases of HZ were observed over the study period, corresponding to an incidence rate of 5·23/1000 person-years (95% CI 5·17–5·29). HZ incidence increased markedly with age up to 84 years (Fig. 1).

Fig. 1. Herpes zoster (HZ) incidence rate with 95% CI by age group – immunocompetent population.
HZ occurred more often in women (6·05/1000 person-years, 95% CI 5·95–6·14) than in men (4·30/1000 person-years, 95% CI 4·22–4·38) and this difference was consistent within each age group (Fig. 2).
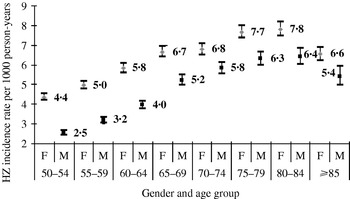
Fig. 2. Herpes zoster (HZ) incidence rate by age group and gender.
In the sample the mean age was 67·9 years (s.d.=10·9) and 61·1% of patients were female. Of all patients, 43·7% suffered from mild to severe pain due to HZ.
Proportion of HZ patients developing PHN
After excluding patients with diabetic neuropathy, epilepsy and recurrent HZ, 25 002 patients were selected, of whom 4884 patients (19·5%, 95% CI 19·0–20·0) and 3415 patients (13·7%, 95% CI 13·2–14·1) developed PHN using the 1-month and 3-month definitions respectively.
With the 1-month definition, the mean age was 71·2 years and 65·4% of patients with PHN were female. Modelling the probability of developing PHN using logistic regression including age and gender as factors, women were found to be significantly more likely to develop PHN, even after adjustment for age. Patients with PHN also tended to be in poorer health: for each category of comorbidity, the proportion of patients having the condition being higher in the PHN population than in the HZ population (Table 1). For example, 31% of patients with HZ had musculoskeletal and connective tissue disease but this proportion reached 43% in patients who developed PHN.
Table 1. Patients' characteristics
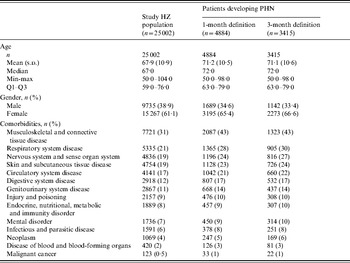
PHN, Post-herpetic neuralgia; HZ, herpes zoster.
The percentage of patients developing PHN (1-month definition) increased with age from 10·3% (95% CI, 9·3–11·4) for ages 50–54 years to 28·9% (95% CI, 27·2-30·7) for ages 80–84 year (Fig. 3).
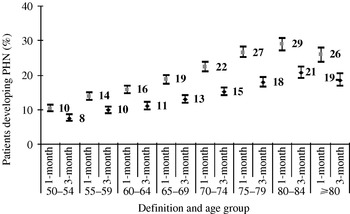
Fig. 3. Proportion of HZ patients developing post-herpetic neuralgia (PHN) by age group and 95% CI.
PHN severity and duration
Thr frequency of severe pain was similar with the two definitions: 39·5% (1 month) and 41·5% (3 months) of patients experienced mild pain and 11·5% (1 month) and 9·4% (3 months) suffered severe pain. The proportion of moderate to severe PHN tended to increase with age (Fig. 4).
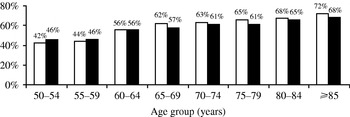
Fig. 4. Proportion of moderate to severe post-herpetic neuralgia (PHN) cases by age group. □, PHN 1 month; ■, PHN 3 months.
The mean duration of PHN was 7·5 months (s.d.=11·9, min-max 0·2–72·4) and 9·0 months (s.d.=12·8, min-max 0·2–70·1) using the 1-month and 3-month definitions respectively. PHN duration increased markedly with severity: patients with mild pain had a mean duration of 5·7 months (95% CI 5·2–6·2) compared to 8·3 months (95% CI 7·7–8·8) for moderate and 10·4 months (95% CI 9·3–11·6) for patients with severe pain using the 1-month definition. The analysis based on the 3-month definition provided similar results with the following mean durations by severity: mild (6·7 months, 95% CI 6·1–7·4), moderate (10·0 months, 95% CI 9·4–10·7) and severe (12·5 months, 95% CI 11·1–14·1).
Economic analysis
Outpatient cost of HZ and PHN management
The mean direct cost of HZ management, excluding hospitalizations, was estimated at £75.63 per episode (95% CI 74·68–76·58).
Patients had on average 1·4 GP visits (s.d.=1·2) due to HZ, resulting in a mean cost of £30.93 (s.d.=33·59), which accounted for 40·9% of the direct costs. Only 2·9% of patients had records of secondary-care visits, mainly to ophthalmologists and physiotherapists, giving a mean cost of £4.18 (s.d.=30·30) per patient overall study population.
Most of the direct costs (53·6%) were attributable to prescribed medications (£40.52), of which 94·5% were due to antivirals, although only 56·3% of patients were prescribed antivirals for HZ. Forty-four percent of the antiviral prescriptions were related to famciclovir, which as the most expensive, accounted for 82% of the antiviral costs. Other medications prescribed for HZ included non-opioid analgesics and TCAs (respectively 39·8% and 15·3% of cases; 2·7% and 0·8% of medication costs).
The cost attributed to the management of HZ sharply increased with the level of pain: patients with severe pain incurred health-care costs, which were more than twice as high as for patients without pain (£124.89, 95% CI 118·55–131·23 vs. £60.15, 95% CI 56·98–61·33) and patients with severe pain incurred GP costs 3·4 times higher than patients with no pain (Table 2).
Table 2. Mean outpatient direct cost per herpes zoster episode (excluding hospitalizations) by severity of pain
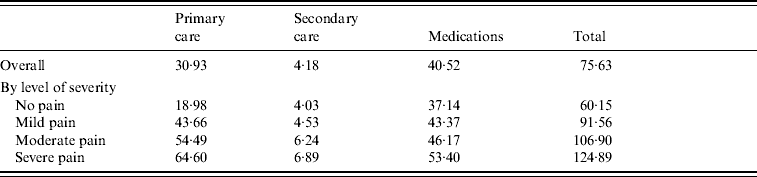
Values are given in £s.
Using the 1-month definition, the average monthly cost of PHN was £41.75 (95% CI 40·74–42·75). Most of the direct costs (63·5%) were attributable to primary-care consultations, with a mean of £26.49 (95% CI 26·02–26·96), and the second source of costs (32·6%, £13.59) related to prescribed medications (95% CI 12·99–14·20).
Most patients were prescribed TCAs (61·8%), but due to their low cost, TCAs only accounted for 15·4% of the costs of medications. About one third of patients received weak opioid analgesics (19·5% of the medication costs). Anticonvulsants, topical analgesics and non-opioid analgesics were prescribed in 20% of patients. Being more expensive, anticonvulsants accounted for 39·7% of the costs of medications whereas topical analgesics and non-opioid analgesics accounted for 24·0% of the costs. Secondary care, by dermatologists, neurologists and anaesthetists, accounted for only 4% of the direct costs.
Patients with severe pain had an average monthly cost 1·4 times higher than patients with mild pain (£51.59 vs. £37.01, Table 3). Mean total outpatient cost per PHN episode (1-month definition) reached £284.38 (95% CI 267·70–301·06).
Table 3. Mean monthly post-herpetic neuralgia costs (excluding hospitalizations) by severity of pain
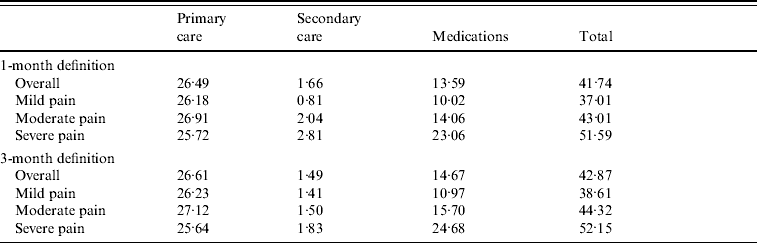
Values are given in £s.
The level of severity drove the costs per PHN episode: cost in patients with severe pain (£519.62, 95% CI 449·78–589·47) was more than three times higher than the cost observed for patients with mild pain (£166.62, 95% CI 148·56–185·18) and more than 1·5 times higher than for patients with moderate pain (£317.97, 95% CI 293·61–342·33).
Monthly costs of PHN were slightly greater using the 3-month definition (£42.78, 95% CI 41·57–43·99). As the duration of PHN was higher using this definition, it resulted in a higher cost per PHN episode (£340.04, 95% CI 319·23–360·85). Cost increase by level of severity was comparable using the two definitions (Table 4).
Table 4. Cost per post-herpetic neuralgia episode (excluding hospitalizations), by severity of pain
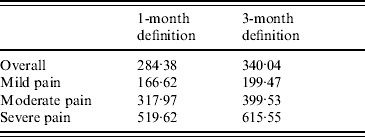
Values are given in £s.
Hospitalization cost of HZ and PHN
HZ caused 2074 hospitalizations in England [15] (mean length of stay 9·9 days). Applying the incidence rate to the population aged ⩾50 years gave an estimate of 88 667 HZ cases in England per year, which led to a mean cost of hospitalisation of £27 per HZ case. The number of hospitalizations for PHN was estimated to be 756 [15] (mean length of stay 11·2 days), which corresponded to additional costs of £57 per PHN episode.
Total cost of HZ and PHN
Combining the costs of outpatient and in-patient care provided a mean total cost of health-care of £103 per HZ case. Depending on the definition of PHN, the mean cost per episode was estimated at £341 (1 month) and £397 (3 months). Most of the costs were incurred in primary care: from 74% for HZ to 86% for the 3-month definition of PHN.
DISCUSSION
This study is the first large study (n=27 225) in many years to provide new incidence data on HZ and PHN and to provide a realistic estimate of its management cost in the United Kingdom. It suggests that the incidence of HZ has remained stable in the United Kingdom over the last 50 years. This is in contrast with recent data from the United States, where an increase has been reported in the overall population [Reference Insinga20] over the past 10–20 years. This has been explained by the ageing population [Reference Insinga20], as age- and sex-adjusted incidence rates have remained stable and may reflect a number of factors including the differences in health-care- seeking behaviour in the United Kingdom, where patients whose rash is mild may not present for medical care.
HZ incidence rates from our study (from 3·44 to 7·29 cases/1000 person-years depending on the age group) were found to be higher than those observed in another recent UK prospective study (n=186) conducted by Scott et al. [Reference Scott12], who reported incidence rates ranging from 0·6 to 4·3. However, the ethnic mix of the Scott study population was not representative of the national population, as it involved a lower proportion of caucasians. This may have affected the incidence rate as it has been suggested that non-whites may be at lower risk of developing HZ [Reference Thomas and Hall21].
As reported by Scott et al. [Reference Scott12], we observed a lower HZ incidence rate in the oldest age group (⩾85 years). This may be due to the fact that 45% of individuals aged ⩾85 years are institutionalized, compared to 26% of patients aged 80–84 years and <17% in the younger age groups [Reference Bebbington, Darton and Netten22]. These patients receiving care in institutions are unlikely to be included in any GP-based database.
Our results confirm that the percentage of patients developing PHN has not decreased since Hope-Simpson's study in 1975 [Reference Hope-Simpson13], in spite of treatment with antivirals for 50% of the population. These findings suggest that antiviral treatment may have no effect on the percentage of patients developing PHN [Reference Alper and Lewis23].
Using retrospective databases has many advantages, which have been listed elsewhere in the literature, but also some drawbacks. For the calculation of HZ incidence we relied on the presence of the code for HZ. It is possible that either some cases were not coded, i.e. not diagnosed or that incorrect codes were used, leading to a misidentification of HZ cases. That said, the pattern of HZ incidence as a function of age is consistent with the prospective study reported by Scott et al. [Reference Scott12] recently.
Females were found to be more likely to develop HZ after adjustment for age. As incidence is computed on the basis of health-care contacts, the health-care-seeking behaviour could explain part of the difference between genders [Reference Opstelten24]. However, Fleming et al. [Reference Fleming25] have also reported a higher HZ incidence in females (28% more cases annually in females after adjustment for age), suggesting that they may indeed have a different immune response to shingles. Moreover, Opstelten et al. [Reference Opstelten24] found that females were more likely to develop HZ after adjustment for age, and comorbidities known to affect the immune system and to be related to HZ.
PHN was identified by reported diagnoses and prescription of specific neuropathic pain medications. This approach, selected because PHN diagnoses may be under-reported in the GPRD, may have resulted in a biased estimation of the true PHN proportion in the HZ population. Nevertheless, results were consistent with previous studies: Hope-Simpson [Reference Hope-Simpson13] reported that 20·5% of patients developed PHN using the 1-month definition and Scott et al. [Reference Scott12] reported that 13·4% of HZ patients developed PHN using the 3-month definition, indicating that overestimation is unlikely. However, differences in PHN patients' identification in these various studies make the direct comparison of these results difficult. Indeed, Scott et al. [Reference Scott12] identified PHN patients as patients with pain at 3 months as defined by a questionnaire specifically developed to assess HZ- and PHN-associated pain (the Zoster Brief Pain Inventory, or ZBPI [Reference Coplan26]). In the Hope-Simpson [Reference Hope-Simpson13] study, PHN was diagnosed if pain continued to be reported for 1 month after HZ rash onset. Furthermore, given the lack of consensus on PHN definition, we proposed to explore both PHN definitions at 1 and 3 months after HZ rash onset. However, recent researches tend to define PHN as pain persisting more than 3 months after rash onset, pain persisting at 1 month being more commonly defined as ‘zoster-related pain’.
This analysis involved the measurement of the severity of pain related to PHN. This is not recorded in the GPRD and was therefore assessed on the basis of prescriptions of pain medication, which were used as a surrogate, leading to a potential underestimation of severity levels. Results were not directly comparable to the results reported by Chidiac et al. [Reference Chidiac27] and Oxman et al. [Reference Oxman14], but this was essentially caused by the difference in study design, i.e. prospective vs. retrospective, prospective studies enabling the use of specific instruments to assess the level of severity. In the study described by Chidiac, severity was assessed on the basis of a questionnaire designed to evaluate perceived pain and 41% of patients developing HZ had severe or very severe pain [Reference Chidiac27], whereas only a minority of the patients selected in our study (3·1%) were considered as experiencing severe pain. The proportion of patients with severe PHN was much lower in our study (11·5% compared to 52% [Reference Chidiac27]). This may be because patients do not consult their GP immediately after the PHN start date; as PHN severity decreases over time, this may result in underestimation of severity. Moreover, the difference can originate from the different definitions used to define PHN and pain severity level: the proportions observed in the study described by Chidiac are based on questionnaires completed by the patients to assess perceived pain whereas our results are based on prescribed medications. Lastly, GPs may be reluctant to prescribe severe pain medications, leaving severe pain inadequately treated or, in case of the more elderly population, be reluctant to prescribe treatments due to intolerance or possible interaction with other medications.
This study provides a recent estimate of the clinical and economic burden of HZ and PHN in the United Kingdom. The cost of HZ was £103 per patient and the cost per PHN episode was estimated at £341 using the 1-month definition and £397 using the 3-month definition, which is comparable to the cost estimated by Edmunds et al. [Reference Edmunds, Brisson and Rose10] (£306, 1998 costs). Davies et al. [Reference Davies28] reported a cost of £770 (1991–1992 £s) per PHN episode for patients attending a tertiary referral centre, who are likely to be more severe than the average patients analysed in the GPRD database. This estimate is comparable to the mean outpatient cost observed in severe patients: £519.62 and £615.55 using the 1- and 3-month definitions. For both HZ and PHN, the level of severity drove the cost: patients with severe pain incurring 2–3 times higher costs than patients with mild pain.
A major drawback is that detailed information related to secondary-care referrals is not available in the GPRD database, meaning that no information is present on medications prescribed by specialists and the number of consultations is unknown. Consequently the associated total cost has probably been underestimated. The extent of this underestimation is difficult to assess. The study by Scott et al. [Reference Scott12] reported a mean HZ cost of £524 over 6 months. However, the results observed in this study (n=96) may not be applicable to the general population.
Finally, the present study aimed to report epidemiological and direct medical cost data and does not consider indirect cost of HZ and its complications. Societal costs of HZ and PHN would include costs associated with productivity loss, which are expected to be substantial. The humanistic burden associated with HZ and PHN, namely the reduction in quality of life associated with both diseases, was not included either. This can be quite substantial especially in the case of PHN with severe pain [Reference Oster1]. Although HZ has a markedly shorter duration than PHN, the pain burden also results in a substantial loss of quality of life and can have a profound effect on patients' daily lives [Reference Katz29]. Therefore, further research would be of interest to estimate accurately the societal cost of HZ and PHN in the United Kingdom and their impact on the quality of life.
Furthermore, as HZ and PHN mainly affect more elderly populations, the burden will be affected by changes in the demographic composition of the United Kingdom, which is set to increase by 25% by 2020.
These data are of interest from both a public health and economic perspective when assessing the impact of a new vaccine able to prevent HZ and PHN, thereby avoiding patient suffering and the use of scarce NHS resources.
ACKNOWLEDGEMENTS
This study is based in part on data from the Full Feature General Practice Research Database obtained under licence from the UK Medicines and Healthcare Products Regulatory Agency. The interpretation and conclusions contained in this study are those of the authors alone. This study was funded by Sanofi Pasteur MSD. i3 Innovus, an independent contract research organization, was contracted to design and conduct the study.
NOTE
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org).
DECLARATION OF INTEREST
None.












