Background
The use of cannabis (marijuana) for symptom relief in people with multiple sclerosis (MS) has been reported to be widespread. In 1998, The US Institute of Medicine recommended that formal clinical studies should be carried out, a view that was echoed by the United Kingdom (UK) House of Lords Science and Technology Select Committee the same year. Clinical trials of a standardised medicinal extract of cannabis (Sativex®, GW Pharma Ltd, Salisbury, Wiltshire, UK) started in 2000. Sativex is formulated from 9-delta-tetrahydrocannabinol and cannabidiol in a 1:1 ratio, and acts as an endocannabinoid system modulator, administered to patients as an oromucosal spray. The development of this medicine has required a large-scale coordinated programme of randomised controlled trials (RCTs) in patients with neurological pathology. During these trials, the efficacy of Sativex over placebo has been demonstrated in patients with intractable peripheral neuropathic pain (Nurmikko et al., Reference Nurmikko, Serpell, Hoggart, Toomey, Morlion and Haines2007), central neuropathic pain (Rog et al., Reference Rog, Nurmikko, Friede and Young2005), and symptoms of MS-induced pain (Rog et al., Reference Rog, Nurmikko, Friede and Young2005) and spasticity (Collin et al., Reference Collin, Davies, Mutiboko and Ratcliffe2007). In addition, most of these studies have shown that Sativex improves sleep quality. In Canada, Sativex was approved and marketed for the management of neuropathic pain in MS in 2005, and for the management of cancer-related pain in August 2007. More recently, it has been licensed for use in the United Kingdom, Spain, Germany and other countries as an add-on treatment for symptom improvement in patients with moderate to severe spasticity due to MS (MHRA Public Assessment Sativex Report, 2010). Upon completion of the above-mentioned RCTs, many patients continued to use Sativex as an unlicensed prescription medicine, whereas others participated in long-term open-label studies, the results of which have shown persisting benefit (Wade et al., Reference Wade, Makela, House, Bateman and Robson2006; Rog et al., Reference Rog, Nurmikko and Young2007). As an unlicensed medicine, the decision to prescribe Sativex for patients was entirely at the discretion of the prescribing doctor, and under their direct responsibility. Patients receiving Sativex in this manner would be expected to be suffering from long-term disease with severe symptoms, and to be poor responders to current MS treatments. These characteristics make treating this group of patients especially challenging. Added to this is the fact that MS sufferers commonly have a range of functional impairments, which can be challenging for carers.
Although the efficacy and safety of Sativex have been demonstrated in a number of RCTs, the results of these studies may not always reflect the wider effectiveness that a medicine shows when used in clinical practice. Patient satisfaction surveys are widely used in determining the long-term impact of a new medicine, and can help to differentiate between those aspects of a chronic condition that may benefit compared with those that do not. As the first ever licensed cannabis-based medicine, understanding the effects of Sativex's long-term use will be crucial for determining future treatment decisions. The current postal survey was therefore designed to determine what benefits, if any, patients and their carers (ie, partner, mother, etc.) perceived from the long-term use of Sativex, prescribed on an unlicensed basis, in addition to providing further information to the specialist teams involved in the everyday management of patients with MS who use a cannabis-based medicine for symptom control.
Methods
The primary objective of the study was:
To collect information describing the effect that long-term Sativex use had on the function of patients.
The secondary objectives were:
-
a) To identify any evidence of changing patterns of Sativex use over time.
-
b) To identify any impact of Sativex use on the carer(s) of MS sufferers.
-
c) To identify whether the use of Sativex impacted on the use of healthcare resources by patients.
-
d) To identify any evidence of diversion of Sativex from its intended use, and to improve the quality of information provided to patients about the use of Sativex.
In order to meet these objectives, a questionnaire was designed in consultation with the UK patient representative organisation, The Multiple Sclerosis Trust, and with the Pain Research Unit at the James Paget University Hospital, Great Yarmouth. The questionnaire survey was then field tested before submission for approval by the Cambridgeshire 4 Research Ethics Committee (UK).
Doctors who had prescribed Sativex to an individual patient at least twice during the previous 16 weeks were identified from a statutory anonymised register of Sativex users held by GW Pharma Ltd under the supervision of the UK Home Office. There was no minimum duration of Sativex dosing stipulated in the entry criteria, as long as subjects had received two prescriptions of Sativex within the stipulated time. The questionnaire was mailed to the prescribers, with an invitation to them to send the questionnaire on to the relevant patients. The introduction to the questionnaire provided information for the patients, explaining the purpose of the study and giving guidance on completion. It was made clear that they were under no obligation what so ever to complete the questionnaire.
Most questions were of a simple multiple-choice style. There were a few questions requiring a free-text answer to enable the subject to provide more detail on a previous answer. The first part of the questionnaire was designed for all patients who had been prescribed Sativex. These were demographic questions on age, diagnosis and symptoms, and then on the duration and pattern of Sativex use and the benefit for the patients’ sleep.
The second part (MS Subgroup) was a set of questions for patients with MS and their carers (as defined by the patient, and included partners, mother, nurses or outside carers). The questions for the patients focused on changes in activities of daily living and also examined the use of medical care including accidents. The final set of questions to carers explored both activities needing their assistance (ie, toileting) and the disturbance of their sleep.
GW Pharma Ltd then gathered and stored data in conformation with the requirements of the Data Protection Act. No data were collected that would permit the identification of the patient.
Results
Demographics: all patients
A total of 218 questionnaires were sent out to UK prescribers, and 124 questionnaires (57%) were returned. The demographics of the respondents are shown in Table 1. The age and gender distribution of the patients closely resembles that seen in more than 1500 patients who took part in the clinical trials programme for Sativex, and it therefore seems likely that they represent a similar population (MHRA Public Assessment Sativex Report, 2010).
Table 1 Demographic characteristics of respondents (n = 124)

MS = multiple sclerosis.
Exposure: all patients
There was a broad variation in the number of daily sprays of Sativex used by patients (see Figure 1). The median daily dose was six daily sprays, and only 27 (22%) patients used more than eight sprays per day.

Figure 1 Number of daily sprays – all patients (n = 124). *Each spray of Sativex contained 2.7 mg delta-9-tetrahydrocannabinol (THC) and 2.5 mg cannabidiol (CBD), minor cannabinoids and terpenoids.
Patients reported on whether their daily use of Sativex had increased, decreased or stayed the same since they had started using it. The majority (88 patients; 71%) of respondents reported that their use had continued at the same dose, whereas 13 (10%) stated that their use had decreased, compared with 23 (19%) patients whose use had increased.
Another survey question asked whether patients had ever shared their medication and three out of 124 admitted to this. However, none had ever lost their medication, which is a common finding in patients overusing or sharing prescribed opiate medication (Bates, Reference Bates2005).
Sleep: all patients
All patients, regardless of the condition for which they were being prescribed Sativex, reported on whether there had been an improvement, no change or deterioration in their sleep. The majority (102 patients (82%)) of patients reported an improvement in sleep (Figure 2a), with ‘much improved’ being reported by 41 (33%) patients since starting Sativex.

Figure 2 Changes in sleep reported by patients since starting Sativex: (a) (n = 122) – all patients and (b) (n = 90) – MS (multiple sclerosis) Subpopulation (Key: +++, very much improved; ++, much improved; +, improved; −, slightly worse; −−, much worse; −−−, very much worse). (c) Night-time disturbance of carers (n = 53) – MS Subpopulation (Key: +++, much less; ++, less; +, a little less; −, a little more; −−, more; −−−, much more).
MS Subgroup
The remainder of the questionnaire was targeted specifically at patients being prescribed Sativex for their MS symptoms and, where appropriate, their caregivers. There were 92 patients in this subgroup (74% of respondents). The median duration of MS was 17.0 years (ranging from three to 40 years) in these patients, implying that they were likely to represent a group with a significant level of disability. Although no formal assessment of this was possible in this survey, the criterion for inclusion in previous clinical trials was intractability of symptoms.
Five questions were posed to patients to reflect any change in utilisation of medical resources, including changes in other anti-spasticity medication, frequency of visits to the doctor, etc. Figure 3 summarises the results of this section of the questionnaire.

Figure 3 Change in the use of healthcare resources and specific functional activities reported by patients – MS (multiple sclerosis) Subpopulation (Key: a, use of other anti-spasticity medication (n = 87); b, frequency of visits to doctor/physiotherapy (n = 88 and n = 80, respectively); c, use of respite care (n = 72); d, accidents requiring medical attention (n = 85); e, tooth brushing (n = 86); f, dressing self (n = 86); g, feeding independently/picking up a drink (n = 84 and n = 83, respectively); h, able to write (n = 85); i, carry a shopping bag (n = 84); j, walking (n = 85); k, stand up (n = 85); l, sitting with comfort/lie in bed with comfort (n = 85 and n = 86, respectively)).
There was an apparent reduction in the reported number of medical and physiotherapy contacts, and most notably a large reduction in the number of accidents requiring medical attention.
The reasons for taking Sativex were defined as spasticity by 52 patients (57%), pain by 31 patients (34%) and to improve sleep by seven patients (8%). The spasticity affected the legs in 91 (99%) patients, trunk in 38 (41%) patients, arms in 36 (39%) patients and hands (36%) in 33 patients. A specific question on ambulation was not included.
The majority of MS patients (76 patients (84%)) reported an improvement in sleep (Figure 2b), with ‘much improved’ being reported by 25 (28%) patients since starting Sativex, of the 90 patients who responded.
In addition to sleep, the MS Subgroup of patients was also asked to report whether there had been improvement, deterioration or no change in their spasticity. A total of 63 (69%) patients reported an improvement in their spasticity since starting on Sativex, and only three (3%) patients reported deterioration. An improvement in other symptoms aside from their spasticity was reported by 55 (60%) patients in the MS Subgroup.
Activities of daily living
Patients were asked to report whether specific functional abilities had changed since they started using Sativex. The changes reported by patients in these activities of daily living are shown in Figure 3.
The most striking improvements were seen in the patients’ reported ability to lie in bed or sit in a chair with comfort, and to stand up. In all activities except carrying a shopping bag, the proportion of patients reporting improvement on Sativex exceeded the proportion of patients reporting deterioration over time. Patients were also asked whether they were able to undertake any activities without the need for help or equipment, and 12 (14%) patients reported that they were now able to do so.
Overall, of the 88 patients who responded, 83 (94%) believed that they had obtained benefit from the use of Sativex.
Caregivers
The final part of the questionnaire for the MS Subgroup was completed by the patients’ caregivers. There were 66 patients with caregivers, of which 53–58 responded. The vast majority of carers (48 carers (87%)) described themselves as a partner, whereas three (5%) were mothers, three (5%) were carers and one (2%) was a nurse (n = 55).
When asked whether they had found night-time care to be more difficult or easier since starting Sativex (Figure 2c), 25 carers (47%) reported that night-time care had substantially improved, and 20 reported (38%) seeing no improvement (n = 53).
Overall, the amount of time spent caring for the person with MS had increased for 9 (16%) carers, decreased for 14 (25%) carers and remained the same for 32 (58%) carers (n = 55).
Carers were also asked whether specific activities had changed since Sativex had started. The results are shown in Table 2. With the exception of toileting, the improvements reported by caregivers substantially exceeded the deterioration reported over time for all activities of daily living. The greatest improvement was reported for transferring, for example moving from wheelchair to bed (25 carers (49%) reported an improvement versus 13 (26%), who reported a deterioration; n = 57).
Table 2 Percentage of carers reporting (n = 57) overall improvement versus deterioration in activities of daily living in people with MS spasticity treated with Sativex – MS Subpopulation
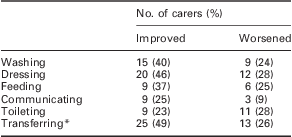
MS = multiple sclerosis.
*For example, moving from wheelchair to bed.
Finally, carers were asked whether there had been an overall benefit from treatment with Sativex, to which the majority (42 carers (72%)) responded that there had been benefit to the patient, and 47 (82%) reported that there had been benefit to them (n = 58).
Discussion
This postal survey adds valuable information about the long-term clinical benefits of Sativex use in the treatment of patients with prolonged, treatment-resistant and disabling disease, as perceived by both patients and their caregivers. The results of the questionnaire report improvements in several key activities of daily living, as well as improvements in sleep quality for both patients and their caregivers. Furthermore, the study adds valuable insight into specific areas of patients’ lives that have improved or remained stable with long-term Sativex treatment, as well as investigating the impact of treatment on caregivers. We believe that these findings provide useful information to add to the cost–benefit discussion for this medicine.
The efficacy and safety of Sativex has been previously demonstrated in several formal clinical studies (Rog et al., Reference Rog, Nurmikko, Friede and Young2005; Reference Rog, Nurmikko and Young2007; Collin et al., Reference Collin, Davies, Mutiboko and Ratcliffe2007) and, more recently, it has been licenced for use in a number of countries in Europe, North America and Australasia as an add-on treatment for MS symptoms, including spasticity and pain (MHRA Public Assessment Sativex Report, 2010). Although data from RCT studies are critical in determining the effectiveness of a new medicine, the tightly controlled nature of these studies, coupled with the relatively short treatment duration and the necessary focus on a single primary outcome measure, can make the results difficult to translate into a ‘real-world’ setting. Here we report on the efficacy of long-term Sativex treatment, as administered on an unlicensed basis before its marketing in 2010, in patients with experience of its prolonged use.
In the MS subset of patients, both patients and their carers reported improvements in the overall severity of spasticity, and in a series of activities of daily living. Notably, daily activities in which patients demonstrated the most improvement were those that reflect the severity of spasticity, including holding a glass, standing up, sitting in a chair with comfort and lying in a bed with comfort. Carers also reported improvements in areas in which they normally assist, including washing, dressing, communicating and transferring (eg, moving from wheelchair to bed). These findings are supported by a previous six-week RCT investigating Sativex use in the treatment of spasticity in MS patients. During this RCT, Sativex was found to be more effective than placebo at alleviating patients spasticity (Collin et al., Reference Collin, Davies, Mutiboko and Ratcliffe2007). Similar findings were also demonstrated in other RCTs with cannabinoid-based medicines, in which patients with MS reported significant improvements in spasticity and pain with cannabinoid treatment (Zajicek et al., Reference Zajicek, Fox, Sanders, Wright, Vickery, Nunn and Thompson2003), with a 12 month follow-on study providing evidence of the long-term efficacy of cannabinoid treatment for spasticity (Zajicek et al., Reference Zajicek, Sanders, Wright, Vickery, Ingram, Reilly, Nunn, Tearer, Fox and Thompson2005). The results of the current investigation are in line with these findings, and add valuable information about the range of activities associated with spasticity, which have improved with Sativex treatment.
Many patients also reported improvements in their nights when using Sativex. This may have been due to reductions in spasms, pain, nocturia or from enhancement of sleep, possibly reflecting some of the various sites of action of cannabinoids. As a result of this, the quality of sleep for caregivers was also reported to improve in this survey. Such reductions in the caregiver burden are known to be associated with an improvement in caregiver quality of life (Buhse, Reference Buhse2008). Similar improvements in sleep quality have been reported in numerous clinical trials of Sativex and other cannabinoid-based medicines (Zajicek et al., Reference Zajicek, Fox, Sanders, Wright, Vickery, Nunn and Thompson2003; 2005; Brady et al., Reference Brady, DasGupta, Dalton, Wiseman, Berkley and Fowler2004; Wade et al., Reference Wade, Makela, House, Bateman and Robson2006; Rog et al., Reference Rog, Nurmikko, Friede and Young2005; 2007), which support the current findings, and add integrity to the results from this postal survey.
Of particular note was the observation made by a substantial proportion of the patients that they had suffered fewer accidents requiring medical attention. This not only provides support for the conclusion that their spasticity had improved, but also that it had done so without the associated weakness that is characteristic of treatment with other anti-spasticity agents. This is in line with the findings from a previous RCT of Sativex in patients with spasticity carried out by Collin et al. (Reference Collin, Davies, Mutiboko and Ratcliffe2007), in which patients reported increased power in the legs, suggesting that the reduction in spasticity with Sativex is not gained at a cost of increasing weakness.
In this survey, the mean duration of treatment with Sativex was 30 months, a period of time over which a certain amount of deterioration in symptoms is expected in MS patients, including in their functional capacity. The fact that deterioration was reported by relatively few patients could suggest that Sativex played a role in the improvements seen, but in the absence of a control group this cannot be confirmed.
The finding that three patients had shared their medicine emphasises the importance of careful patient selection, education and monitoring of Sativex use. Previous long-term studies of Sativex have shown that dose escalation is rare, and thus any dose increases should be critically assessed.
The overall 57% response rate to this postal survey is considered to be reasonable (Cull et al., Reference Cull, O'Connor, Sharp and Tang2005). As the questionnaire was distributed to the patients by the prescribers, there is no way to tell whether non-responding patients received a questionnaire, and thus we cannot investigate possible bias in the respondents. Although higher response rates are considered to be desirable, there is no clear evidence to suggest that higher response rates result in more reliable outcomes (American Association for Public Opinion Research, 2008). The responses from both patients and their carers confirmed the activities of daily living in which patients were obtaining benefit. These findings suggest that the maintenance of a response to treatment was genuine, and therefore that the patients could be deemed to be responding to treatment with long-term use. By definition, in using Sativex they had not adequately responded to previous therapy, and the inference is that they were able to tell whether or not they were receiving benefit from treatment.
Also of importance was the fact that the vast majority of caregivers reported that, because of Sativex treatment, there had been a benefit to them. This suggests a reduction in the caregiver's burden, which could also impact on their well-being and also the patients (Buhse, Reference Buhse2008). Taken together, these findings suggest a promising clinical benefit with long-term Sativex treatment, both to patients and their carers.
Limitations
Long-term efficacy of a medicine can only be convincingly shown in the setting of RCTs and the results of a questionnaire survey will always be less robust. Inevitably, patients taking a medicine in the course of usual medical care are self-selected. However, patient satisfaction surveys are widely used in determining the long-term impact of a new medicine or other aspects of healthcare, and can help to differentiate between those aspects of a chronic condition that may benefit compared with those that do not. The response rate of 57% was disappointing, but the overall sample size, at greater than 100, is large enough to provide a worthwhile volume of data. It is not possible to conclude statistical significance with the approach used here, but these observations are hypothesis-generating for future studies.
Conclusion
In conclusion, this survey confirms the results of other surveys and extension studies in showing Sativex to be associated with maintenance of benefit to the quality of life of both patients with MS and their carers, in a chronic condition where progressive deterioration is the norm. In corroboration with a previous RCT, the survey showed that the improvement in spasticity with Sativex was not associated with the increased weakness coupled with other anti-spasticity treatments, a promising finding. The results also show that the use of Sativex may be associated with a reduction in the use of other healthcare resources, and adds to the evidence of meaningful efficacy from placebo-controlled, randomised, double-blind clinical studies.
Acknowledgements
All costs for the study were covered by GW Pharma Ltd, for example those incurred through questionnaire design and printing. The prescribing Doctors, including Dr Notcutt, were paid by GW Pharma Ltd on a per patient basis for each questionnaire that they sent out. We would like to thank Christine Jones, previously Chief Executive of the Multiple Sclerosis Trust, for help in constructing the questionnaire; also Cheryl Phillips, Research Coordinator at the Pain Research Unit, for her invaluable assistance at the study site. We are grateful to Paula Barrows, Clinical Coordination Manager, of GW Pharmaceuticals for help in organising data collection.









