Obesity has now become a major global problem that is associated with risk of many non-communicable diseasesReference Pi-Sunyer1–Reference Must, Spadano, Coakley, Field, Colditz and Dietz3. Although obesity is related to disease riskReference Stevens, Cai, Pamuk, Williamson, Thun and Wood4, some studies suggest that the pattern of body fat distribution is a more important determinant than general obesityReference Wei, Gaskill, Haffner and Stern5–Reference Pi-Sunyer7. Abdominal obesity has also been shown to be associated with increased risk of overall mortality in many populationsReference Prineas, Folsom and Kaye8. Despite some recent studies proposing waist circumference (WC) as an indicator for central fat accumulationReference Pouliot, Despres, Lemieux, Moorjani, Bouchard and Tremblay9, Reference Reeder, Senthilselvan, Despres, Angel, Liu and Wang10, there is no universal agreement regarding the use of WC at presentReference Esmaillzadeh, Mirmiran and Azizi11 and waist-to-hip ratio (WHR) is the most commonly used index of central fat distribution12. Abdominal obesity is reported in 36% of adult women in GreeceReference Kapantais, Tzotzas, Ioannidis, Mortoglou, Bakatselos and Kaklamanou13; according to reports, 65% of OmanianReference Al-Riyami and Afifi14 and 55% of Indian womenReference Beegom, Beegom, Niaz and Singh15 are centrally obese.
Although obesity has strong genetic determinants, its increasing prevalence in populations around the world suggests that environmental factors are promoting or exacerbating the problem. Some possible associations of obesity with factors such as socio-economic status, gender, marriage, physical activity and educational level, among others, are mentioned in different populationsReference Roos, Lahelma, Virtanen, Prattala and Pietinen16–Reference Paeratakul, Lovejoy, Ryan and Bray19. In most studies the prevalence of central fat accumulation is higher in women than men. In Iran, it has been reported that 67% of women and 33% of men older than 20 years are centrally obeseReference Azadbakht, Mirmiran and Azizi20. A study of secular trends in central fat accumulation in Iran showed a 6% increase in central adiposity among men and a 9% increase among women between 1998 and 2002Reference Azizi, Azadbakht and Mirmiran21.
Almost all of the previous studiesReference Azizi, Azadbakht and Mirmiran21–Reference Azizi, Esmaillzadeh, Mirmiran and Ainy24 conducted in Iran used the World Health Organization (WHO) cut-off points to determine central fat accumulation and no previous studies have used the specific cut-off pointsReference Mirmiran, Esmaillzadeh and Azizi25 suggested for Tehrani adults. Therefore, the present study was performed to investigate the correlates of central fat accumulation based on the optimal anthropometric cut-off values of Tehrani adults in a representative population of women.
Subjects and methods
Subjects
In this cross-sectional study, 926 women aged 40–60 years living in different districts of Tehran were selected by cluster random sampling. An A to Z map of Tehran, called Rahyab-e-Tehran and published by the geographic information centre of the municipality of Tehran, was used for sampling. The book contains a map of Tehran divided into 185 pages. We divided each page into 30 blocks: five sections horizontally and six sections vertically. From among 5550 blocks in the whole book, 50 blocks were randomly chosen with 1460 individuals totally. After excluding subjects who had at least one missing variable, 926 women were included in the present analysis. To make sure that the sample was representative of the general population, the age groups of the subjects were compared with those of Tehran and Iran’s population. The age distribution of subjects in this study is similar to that of the urban population in Tehran and Iran.
Anthropometry
All anthropometric measurements were made according to the WHO protocol26. Weight was measured using digital scales while subjects were minimally clothed and without shoes, and recorded to the nearest 100 g. Height was measured to the nearest 1cm using a tape meter while subjects were in a standing position without shoes, with their shoulders in a normal position. WC was measured at the narrowest level, and hip circumference at the maximal level, over light clothing, using an unstretched tape meter without any pressure to the body surface, and was recorded to the nearest 0.1 cm. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2). WHR was computed as WC divided by hip circumference. The cut-off for WHR was determined as ≥0.84 based on the suggested cut-offReference Mirmiran, Esmaillzadeh and Azizi25 for Tehrani women, adjusted for their age group. We used normative threshold values for all anthropometric measures by assessing only the youngest adults, because anthropometric indicators such WC and cardiovascular risk factors increase with age. Accordingly, establishing the thresholds with values from any subpopulation is necessaryReference Dobbelsteyn, Joffres, Maclean and Flowerdew27. On the other hand, a previous study in Iran showed that the so-called ‘action level 2’ cut-off point of WC had low predictive value for cardiovascular risk factors in Tehrani womenReference Esmaillzadeh, Mirmiran and Azizi28.
Dietary intake assessment
Dietary intake was assessed by means of a semi-quantitative food-frequency questionnaire (FFQ). All the questionnaires were completed by trained dietitians with at least 5 years’ experience in the Nationwide Food Consumption Survey projectReference Kimiagar, Ghaffarpour, Houshiar-Rad, Hormozdyari and Zellipour29. The FFQ consisted of a list of foods with a standard serving size. Participants were asked to report their frequency of consumption of each food item during the previous year on a daily (e.g. bread), weekly (e.g. rice, meat) or monthly (e.g. fish) basis. To assist the subjects to report accurately, household utensils were used. The questionnaires were validated 12 years ago in the Nationwide Food Consumption Survey project, which has been reported in Farsi30. We revalidated them with 16 families before this study was begun (unpublished data). Standard reference tables were used to convert household portions to grams for computerised analysisReference Ghaffarpour, Houshiar-Rad and Kianfar31. Following coding of diaries, the dietary recall form was linked to a nutrient database (Nutritionist III) and nutrient intakes were calculated from the quantities of foods consumed.
Lifestyle factors
Information about age, occupation, medication use, marriage, parity, menopausal status, smoking and drinking coffee was obtained using a pre-tested questionnaire. To ascertain physical activity status a validated questionnaireReference Craig, Marshall, Sjostrom, Bauman, Booth and Ainsworth32 was used. Depression was assessed by applying the Beck questionnaireReference Beck33.
Statistical analysis
Associations between lifestyle factors and central adiposity were assessed by logistic regression modelling. Lifestyle factors were categorised as follows. Occupation: unemployed, retired, employed; parity: 0, 1–2, ≥3; menopausal status: postmenopause, premenopause; smoking: non-smoker, smoker; drinking coffee: yes, no; physical activity: light, moderate, heavy; depression: depressed, mildly depressed, normal.
Logistic regression analysis adjusted for age and BMI was used to determine the correlates of central fat accumulation. WHR was dichotomised at a pre-selected cut-off based on the suggested cut-off for Tehrani womenReference Mirmiran, Esmaillzadeh and Azizi25. Subjects were classified on the basis of percentile cut-off points of nutrient intakes: <25, 25–50, 50–75 and >75. Only those nutrients which had a significant correlation with WHR were entered into the model. All nutritional and non-nutritional factors were simultaneously entered into the model. Stepwise multiple linear regression analysis was used to determine the predictors of WHR. Analysis of covariance was employed to compare the mean values of WHR in different lifestyle groups and the mean values of vegetable and dairy consumption in centrally obese and normal women with adjustments for BMI and age. To reduce the data from the FFQ, factor analysis was used and then the association between each component and central fat accumulation was assessed by Pearson correlation. The ethical committee of the National Nutrition Research and Food Technology Institute of Shaheed Beheshti University of Medical Sciences approved the proposal of this study, and informed written consent was obtained from all women.
Results
Mean and standard deviation of age, weight, height, BMI, WC and WHR of the women is shown in Table 1.
Table 1 Characterstics of Tehrani Women

SD – standard deviation.
Table 2 shows the possibility of being centrally obese in different groups based on lifestyle characteristics. The subjects who were less physically active had more chance of being centrally obese compared with the reference group. Unemployment, marriage, menopause and parity were also associated with central fat accumulation.
Table 2 Odds ratio (OR) and 95% confidence interval (CI) for having waist-to-hip ratio ≥0.84 according to different lifestyle factors

*All independent variables were entered simultaneously into the analysis and adjusted for cholesterol, vitamin C, calcium, vitamin B6, fat intake, body mass index, medication use and age.
† P < 0.05.
Women with light physical activity had a higher WHR than women with heavy physical activity (0.85 ± 0.03 vs. 0.80 ± 0.02, P < 0.05). Postmenopausal women had higher WHR than premenopausal women (P < 0.01). Women with three or more live births also had higher WHR compared with the two other groups (P < 0.01). WHR was predicted as follows:
The possibility of being centrally obese in different groups of nutrient intake is shown in Table 3. Mean values of WHR, with adjustments for age and BMI, in different percentiles of fat and vitamin C intake is shown in Fig. 1.
Table 3 Odds ratio (OR) and 95% confidence interval (CI) for having a waist-to-hip ratio ≥0.84 according to different nutrient intakesFootnote *

* Nutrients are categorised according to percentiles: <25, 25 – 50, 50 – 75, >75.
† All independent variables were entered simultaneously into the analysis and adjusted for physical activity level, depression, smoking, coffee consumption, menopausal status, marriage, parity, age, medication use and body mass index.
‡ P < 0.05.

Fig. 1 Waist-to-hip ratio (WHR) in women according to level of vitamin C and fat intake. Values are means with standard error shown by vertical bars. Women in the first percentile of vitamin C and fourth percentile of fat intake had higher WHR (P < 0.01) than those in the fourth percentile of vitamin C and first percentile of fat intake
The dietary intake of the women was factor-analysed into eight components, as shown in Table 4. Among the components, only the dairy group was correlated with WHR (r = −0.2, P < 0.05).
Table 4 Different components of the women’s diet
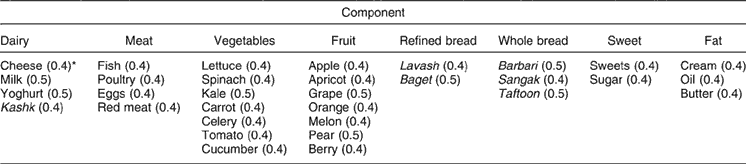
*Loading factor.
Discussion
By conducting a systematic epidemiological analysis, we have revealed the factors associated with central fat accumulation in a representative sample of Tehrani women. The appropriate adjustments for confounders in the present study, as well as use of the suggested cut-off points for Tehrani women to determine central fat accumulation, increased the reliability of our results.
Ageing is one of the determinants of WHR. As ageing is unavoidable, attention to the other determinants such as BMI is necessary. The lifestyle factors related to WHR were very similar to those for BMI, most likely because of the correlation between these two factors. In the present study, subjects who were least active were more likely to have WHR above the suggested cut-off, in the line with previous studiesReference Al-Riyami and Afifi14, Reference Han, Bijnen, Lean and Seidell34. This indicates that increased abdominal fat follows inactivity, which provides important information for health promotion and preventing adiposity. Holcomb et al. also showed the ability of increased daily physical activity in minimising age-related increases in abdominal obesityReference Holcomb, Heim and Loughin35. Depression, which may be a consequence of industrial life, is increasing nowadays. In the present study the likelihood of being centrally obese was higher in depressed women than in healthy women. Therefore, paying attention to mental health is important for preventing central fat accumulation. Unemployed women had higher odds ratio of being centrally obese in this study. Doing repeated housework all days and having no distinct budgetary control may be the causes of depression in housewives and progress the central fat accumulation. On the other hand, exposure to food at home may explain this association. Employed women also have higher economic status in most cases. Previous studies showed higher obesity rate among people of lower socio-economic levelReference Kang, Ju, Park, Kwon, Im and Paek36–Reference Moreira and Padrao38. Smokers in the present study had higher probability of being centrally obese. A longitudinal study has shown that smokers gain weight more on their waist and less on their hips than predicted from gain in body mass, thus they have a gain in WHRReference Shimokata, Muller and Andres39. Other research showed that women who continued to smoke developed significantly higher WHR than those who stopped smokingReference Lissner, Bengtsson, Lapidus and Bjorkelund40. Han et al Reference Han, Bijnen, Lean and Seidell34. showed that smoking cessation reduces the likelihood of being centrally obese.
After adjusting for the effect of age, menopausal women had greater tendency to be centrally obese, which may contribute to the change in sex hormone levels in postmenopausal women. Lipowicz et al Reference Lipowicz, Gronkiewicz and Malina41. showed that after age, marriage is the most important predictor of overweight and obesity. In the current study, the likelihood of being centrally obese was higher in married women; changes in dietary patterns and hormone levels, especially a gain in weight after each pregnancy or lactation, may be the reason. Moreover, we found that multiparous women had also higher WHR, similar to a previous reportReference Han, Bijnen, Lean and Seidell34.
Among nutrient intakes, vitamin C, calcium and fat were associated with central fat accumulation in our study. Vitamin C as well as calcium can reduce fat absorption and may reduce abdominal adiposity. Maskarinec et al. showed that higher fat intake predicted higher BMI (general obesity)Reference Maskarinec, Takata, Pagano, Carlin, Goodman and Le Marchand42 but there are few studies regarding fat and central obesity. Mace et al. reported that dietary fat and fat types should be further studied as early determinants of obesityReference Mace, Shahkhalili, Aprikian and Stan43. After analysing the dietary components, only the dairy group showed a negative significant correlation with WHR in the present study. Previous worksReference Garrow, Webster, Pearson, Pacy and Harpin44–Reference Mirmiran, Esmaillzadeh and Azizi46 have also shown a negative correlation between dairy consumption and BMI. The mechanism by which milk consumption affects obesity indices is not accurately known. Most studiesReference McCarron, Morris, Henry and Stanton47, Reference Fleming and Heimbach48 have cited calcium as a responsible factor. So, an inverse relationship has been suggested between calcium intake and body weight and body fat mass in various ways. Its simple effect is the inhibition of fat and fatty acid absorption. However, this is not the major cause; it seems that the major effect of calcium on body weight is mediated by its effects on controlling intracellular calciumReference Welberg, Monkelbaan, de Vries, Muskiet, Cats and Oremus49. Evidence has shown that the product of agouti, a gene that is expressed in human adipocytes, stimulates calcium uptake into the cells which, by its effect on lipolysis and lipogenesis, causes the deposition of fat in adipocytes. This product increases fatty acid synthetase activity and inhibits lipolysis. By a calcium-dependent mechanismReference Comuzzie and Allison50, vitamin D stimulates calcium entrance into the cell and inhibits lipolysis, so decreasing plasma insulin level by dietary calcium is also mentioned as another reasonReference Shi, Dirienzo and Zemel51. Other factors in addition to its calcium content may play a role in the anti-obesity effect of milk. Trans fatty acids, conjugated linolenic acidReference Awad, Bernardis and Fink52, Reference Belury and KempaSteczko53, protein and bioactive componentsReference Pihlanto-Leppale, Koskinen, Piilola, Tspasela and Korhonen54 have been mentioned in some reports. Rosell et al. also showed an inverse association between sagittal abdominal obesity and calcium intake in a cross-sectional studyReference Rosell, Johansson, Berglund, Vessby, de Faire and Hellenius55.
Using suggested cut-off points for Tehrani women to determine the central fat accumulation was a positive point of this study; the use of cross-sectional data was the principal limitation. Moreover, obesity is a heterogeneous disease and besides nutritional and non-nutritional associations, other factors such as heredity and some other environmental factors that we cannot capture in our analysis must be considered. Although the subjects of this study were a representative sample of Tehrani women, the multi-ethnic nature of our country reduces the possibility of extrapolating of our findings to the whole country. This point emphasises the necessity of conducting similar studies in various regions of Iran. However, a recent study in the north of Iran showed similar associated factors for central obesity. With respect to its findings, low level of activity and education, parity, family history of obesity, marriage at earlier age and ageing were responsible for central obesityReference Hajian-Tilaki and Heidari56.
Therefore, adverse fat distribution is associated with increasing age, unemployment, marriage, parity and poor lifestyle factors including low physical activity, smoking, depression, low intake of vitamin C and calcium, and high fat consumption. The associations may be different in different populations, so using an appropriate tool for diagnosing central fat accumulation and considering its determinants are important for health promotion. Thus lifestyle modifications such as smoking cessation, more physically active lifestyle, avoidance of depression, adequate intake of vitamin C and calcium and less consumption of fat should be encouraged to achieve a healthier body shape.
Acknowledgements
Sources of funding: School of Nutrition, Shaheed Beheshti Medical University, Tehran, Iran.
Conflict of interest declaration: None of the authors had any personal or financial conflicts of interest.
Authorship responsibilities: L.A. and A.E. designed the study, collected and analysed the data, and wrote the manuscript.
Acknowledgements: We thank the participants of the study for their enthusiastic support.









