Clinical Implications
-
• Significant unmet needs exist in the treatment of major depressive disorder, such as suboptimal efficacy and residual cognitive dysfunction.
-
• A paradigm shift from the traditional monoamine therapeutics to approaches integrating glutamatergic function has occurred recently in antidepressant research, and has been especially fueled by the surprising rapid and sustained antidepressant effect of ketamine.
-
• We review the evidence that glutamate neurotransmission can be modulated indirectly by the 5-HT system through the 5-HT1A, 5-HT1B, 5-HT3, and 5-HT7 receptors, and discuss the therapeutic potential of a multimodal approach, combining one or more 5-HT receptor mechanisms with 5-HT reuptake inhibition.
-
• We review the available information for the two multimodal compounds vortioxetine and vilazodone, which are examples of this approach.
Introduction
Over the past 50 years, pharmacological treatments for major depressive disorder (MDD) have evolved from the older tricyclic antidepressants and monoamine oxidase inhibitors, to selective serotonin (5-HT) reuptake inhibitors (SSRI) and serotonin and norepinephrine (NE) reuptake inhibitors (SNRIs). In recent years, antidepressant combination therapies with multifunctional pharmacologic mechanisms have been used to enhance therapeutic outcomes.Reference Stahl 1 Some combinations include an SSRI plus the 5-HT1A receptor and β adrenergic receptor antagonist pindolol,Reference Artigas, Adell and Celada 2 or SSRIs augmented with atypical antipsychotics.Reference Nelson and Papakostas 3 Despite these therapeutic evolutions, significant unmet needs still exist in treating depression, including improving suboptimal treatment response and remission rates, and cognitive impairments in domains such as memory, attention, executive function, and speed of processing.Reference Austin, Mitchell and Goodwin 4 , Reference Lee, Hermens, Porter and Redoblado-Hodge 5 Moreover, some cognitive disturbances may predict the development of mood disorders,Reference Mathews and MacLeod 6 and furthermore may persist beyond remission.Reference Hasselbalch, Knorr and Kessing 7 Since cognitive dysfunction in depression contributes significantly to disability in some patients,Reference Naismith, Longley, Scott and Hickie 8 its alleviation is an important goal.
The glutamate system is the major excitatory neurotransmitter system in the brain and is essential for cognitive processing. In depressed patients, neurochemical assessments have found increased basal glutamate levels in serum or plasma,Reference Altamura, Mauri and Ferrara 9 – Reference Maes, Verkerk, Vandoolaeghe, Lin and Scharpe 11 though changes in its levels in cerebrospinal fluidReference Levine, Panchalingam, Rapoport, Gershon, McClure and Pettegrew 12 , Reference Pangalos, Malizia and Francis 13 and brain tissueReference Francis, Poynton and Lowe 14 , Reference Hashimoto, Sawa and Iyo 15 are somewhat inconsistent. Recent studies using magnetic resonance spectroscopy (MRS) in depressed patients have generally found reductions in GLX, a combined measure of glutamate and glutamine, possibly suggesting that the total glutamatergic pool available for synaptic and metabolic activities is reduced in depression.Reference Yuksel and Ongur 16 However, studies that have directly measured glutamate using MRS have also found inconsistent results, with some groups finding increases, decreases, or no change in glutamate concentrations.Reference Yuksel and Ongur 16 There is also evidence from studies of post-mortem brain tissue in depressed patients or suicide victims for altered expression of N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors.Reference Beneyto and Meador-Woodruff 17 – Reference Mathew, Manji and Charney 20 Given the complexity of glutamatergic neurotransmission and the diversity of these results, it is difficult to come to a definitive conclusion on the role of glutamate in the etiology of major depression at this time. In the future, information on functional single nucleotide polymorphisms related to the glutamate system may provide another valuable method of examining glutamate's role in this disease.
Nonetheless, interest in the role of glutamate in depression is quickly accreting, primarily due to the observation that the noncompetitive NMDA receptor antagonist ketamine engenders a fast and relatively long-lasting antidepressant effect.Reference Kendell, Krystal and Sanacora 21 This observation has prompted a new focus in antidepressant development toward integrating glutamatergic function,Reference Sanacora, Treccani and Popoli 22 leading to the suggestion of a wide range of glutamate targets for the treatment of depression.Reference Hashimoto 23 , Reference Skolnick, Popik and Trullas 24
5-HT neurotransmission is regulated both by the serotonin transporter (SERT),Reference Schloss and Williams 25 which has been a target of antidepressants for the past 30 years, and by modulation via 5-HT receptor subtypes,Reference Smythies 26 some of which (such as the 5-HT1A receptor) may be independent therapeutic targets for the treatment of depression.Reference Artigas 27 A substantial body of data shows that, in addition to modulating 5-HT neurotransmission, multiple 5-HT receptor subtypes can also modulate glutamate neurotransmission. This may be reflected in results from a recent preclinical study, which found that ketamine's fast antidepressant activity was abolished by 5-HT depletion,Reference Gigliucci, O'Dowd and Casey 28 suggesting that these effects may be serotonin-dependent. Thus, there may be an opportunity to integrate monoamine and glutamate strategies for treating depression. A new class of multimodal antidepressants has emerged, which, in addition to inhibiting the SERT, also modulate 5-HT receptors,Reference Nutt 29 , Reference Stahl, Lee-Zimmerman, Cartwright and Morrissette 30 and may represent an example of this integrative strategy.
In this review, we summarize the current knowledge of putative glutamatergic antidepressants, 5-HT receptor-mediated glutamate modulation, and current evidence that multimodal serotonergic antidepressants with indirectly modulating roles on glutamate transmission are active in treating lowered mood and impaired cognition.
Antidepressant Effects by Modulation of Glutamate Transmission
The glutamate receptors are divided into two major families: ionotropic and metabotropic glutamate receptors (mGluRs). The ionotropic family includes NMDA, AMPA, and kainate receptors. The metabotropic family consists of Group I receptors (mGluR1 and mGluR5), which potentiate both presynaptic glutamate release and postsynaptic NMDA currents, and group II (mGluR2 and mGluR3) and Group III receptors (mGluR4, mGluR6, mGluR7, and mGluR8), which in general suppress glutamate function.Reference Javitt, Schoepp and Kalivas 31 , Reference Li, Lee and Liu 32 Glutamate receptors are widely expressed in the brain, and some of them have been implicated in the treatment of depression.Reference Catena-Dell'Osso, Fagiolini, Rotella, Baroni and Marazziti 33 Preclinical and clinical compounds acting via these targets and showing potential antidepressant activity are listed in Table 1.
Table 1 Examples of glutamatergic compounds with antidepressant or antidepressant-like properties

Over-activation of extrasynaptic NMDA receptors is one of several hypothesized glutamate-related pathophysiologies for depression.Reference McCarthy, Alexander and Smith 34 In support of this idea, the noncompetitive NMDA receptor antagonist ketamine at a single i.v. dosing shows rapid (∼4 h) antidepressant effect that is sustained for up to 7 days in therapy-resistant depressed patients.Reference Zarate, Singh and Carlson 35 This rate of onset is extremely fast compared to the 2-3 weeks that approved antidepressants require. A single infusion of a subtype selective NMDA NR2B antagonist traxoprodil has shown a robust separation from placebo in treatment-resistant depression (60% vs 20% response) with sustained effects up to 1 week.Reference Preskorn, Baker and Kolluri 36 However memantine, a use-dependent NMDA receptor antagonist, has not demonstrated the same efficacy as ketamine, though it was not tested in the same paradigm as ketamine.Reference Zarate, Singh and Quiroz 37 Part of the mechanism for the antidepressant effect of ketamine may involve disinhibition of pyramidal cell firing as a result of the antagonism of NMDA receptors located on interneurons.Reference Anticevic, Gancsos and Murray 38 However, it remains to be seen whether the NMDA receptor blockade alone mediates this fast antidepressant activity.
In support of a role for AMPA receptors in treating depression, preclinical studies suggest that ketamine exerts its antidepressant-like effect through AMPA receptors,Reference Maeng, Zarate and Du 39 and that fast action is accompanied by rapid neuronal and synaptic adaptation.Reference Li, Lee and Liu 32 , Reference Tripp, Oh, Guilloux, Martinowich, Lewis and Sibille 40 It is widely believed that neuroadaptive changes represent a key event during antidepressant treatment, and may play a role in the delayed onset of efficacy in traditional antidepressants.Reference Murphy, Norbury, O'Sullivan, Cowen and Harmer 41 , Reference Norbury, Mackay, Cowen, Goodwin and Harmer 42 Thus, ketamine's rapid effects on neuroadaptation may be a key mechanism in its antidepressant effects, and may converge with the general actions of antidepressant treatments suggested in the past decades. Furthermore, the AMPA receptor potentiator aniracetam has shown an antidepressant-like profile.Reference Knapp, Goldenberg and Shuck 43 , Reference O'Neill, Bleakman, Zimmerman and Nisenbaum 44 However, the clinical benefit of AMPA receptor potentiation in depression remains unsubstantiated.
Lamotrigine, a modulator of glutamate release via its action on sodium and calcium channels, is approved for relapse prevention in bipolar disorder in the United States, and may have antidepressant properties in unipolar patients.Reference Hahn, Gyulai, Baldassano and Lenox 45 Additionally, it may accelerate the rate of onset in combination with traditional antidepressants.Reference Obrocea, Dunn and Frye 46 , Reference Normann, Hummel and Scharer 47 Riluzole, which acts to rebalance glutamate levels by enhancing glutamate transport in astrocytes, has shown efficacy in treatment-resistant and bipolar depression.Reference Zarate, Payne and Quiroz 48 , Reference Sanacora, Kendell, Fenton, Coric and Krystal 49 Further examples of targets in the glutamate system with antidepressant-like implications include mGluR2/3 and mGluR5 antagonists or negative allosteric modulators.Reference Belozertseva, Kos, Popik, Danysz and Bespalov 50 – Reference Higgins, Ballard and Kew 52
Thus, although there is evidence that drugs that negatively modulate some aspects of glutamate neurotransmission have antidepressant-like effects,Reference Belozertseva, Kos, Popik, Danysz and Bespalov 50 – Reference Higgins, Ballard and Kew 52 there is also evidence that increasing other aspects of glutamate signaling can have antidepressant-like effects.Reference Knapp, Goldenberg and Shuck 43 , Reference O'Neill, Bleakman, Zimmerman and Nisenbaum 44 It remains to be seen which variables are the true mediators of these effects. In comparison, the prominent role of glutamatergic neurotransmission in cognitive function is better understood. Antagonism of NMDA receptorsReference Riedel, Platt and Micheau 53 as well as other experimental manipulations that reduce aspects of glutamatergic neurotransmission, such as antagonism at AMPAReference Teixeira, Pomedli, Maei, Kee and Frankland 54 or mGlu5 receptors,Reference Homayoun, Stefani, Adams, Tamagan and Moghaddam 55 are known to consistently impair function across a range of cognitive domains. Accordingly, the glutamatergic neurotransmitter system has become a common target in developing cognition-enhancing drugs,Reference Moghaddam 56 with the broad theme that increasing synaptic glutamate neurotransmission, for example using positive allosteric modulators at AMPA (AMPAkinesReference Hamlyn, Brand, Shahid and Harvey 57 ), mGluR5 (CDPPBReference Fowler, Walker and Klakotskaia 58 ), or NMDA receptors (D-cycloserineReference Ozawa, Kumeji, Yamada and Ichitani 59 ), improves cognitive function in rodent models. However, improving mood and cognition by directly modulating glutamatergic neurotransmission may be difficult, as excessive glutamatergic activation can lead to excitotoxic effectsReference Choi, Maulucci-Gedde and Kriegstein 60 and cognitive impairment.Reference Zajaczkowski, Frankiewicz, Parsons and Danysz 61 Furthermore, the near-ubiquitous expression of glutamatergic receptors in the brain may hamper the specificity of drug development.
Thus, a strategy to indirectly modulate glutamatergic neurotransmission in selected brain regions may be more advantageous. A recent preclinical report demonstrated that 5-HT depletion abolished ketamine's antidepressant-like activity, suggesting that 5-HT plays an important role in its action.Reference Gigliucci, O'Dowd and Casey 28 Furthermore, multiple 5-HT receptors modulate glutamate neurotransmission. Taken together, these data make it reasonable to explore a strategy in which 5-HT receptor modulation can be used to alter glutamate neurotransmission in a manner that may improve both mood and cognitive function.
Modulation of Glutamate Transmission by 5-HT Receptors
Here we discuss four 5-HT receptors known to be involved in the action of multimodal antidepressants that have been approved or are in the approval process, and which have the potential to modulate the glutamate system based on their localization and function.
5-HT1A receptors
The 5-HT1A receptor is an inhibitory autoreceptor or heteroceptor located on serotonergic and other neurons, whose activation typically results in suppression of neuronal activity. The main function of presynaptic autoreceptors localized in the midbrain raphe nuclei is to self-regulate the function of the serotonergic system.Reference Sprouse and Aghajanian 62 Desensitization of these autoreceptors is believed to play an important role in the onset of action of SERT inhibitors.Reference Blier and de Montigny 63 , Reference El Mansari, Sanchez, Chouvet, Renaud and Haddjeri 64 The antidepressant potential of 5-HT1A receptor agonism or partial agonism has been studied in both preclinical and clinical settings.Reference Kennett, Dourish and Curzon 65 , Reference Robinson, Rickels and Feighner 66
As postsynaptic heteroreceptors, 5-HT1A is localized in the hippocampus, septum, amygdala, and corticolimbic areas.Reference Martinez, Hwang and Mawlawi 67 , Reference Santana, Bortolozzi, Serrats, Mengod and Artigas 68 Based on immunocytochemical studies, the 5-HT1A receptor is expressed in both pyramidal cells and GABAergic interneurons in the cortex and hippocampus.Reference Aznar, Qian, Shah, Rahbek and Knudsen 69 Unlike presynaptic 5-HT1A receptors, which mainly act through inhibition of adenylate cyclase, postsynaptic 5-HT1A receptors exert their inhibitory action through G protein-coupled inwardly rectifying K+ channels.Reference Luscher, Jan, Stoffel, Malenka and Nicoll 70 Due to the inhibitory nature of GABAergic interneurons, stimulation of 5-HT1A receptors located on interneurons can paradoxically increase cortical pyramidal cell firing, although higher doses can suppress it, probably due to the action of 5-HT1A receptors on the pyramidal cells.Reference Llado-Pelfort, Santana, Ghisi, Artigas and Celada 71 – Reference Wang, Zhang and Liu 73 Similarly, 5-HT1A receptor stimulation resulted in inhibition of GABAergic interneurons in the hippocampus.Reference Levkovitz and Segal 74 Thus, based on the localization of 5-HT1A receptors on both GABA and glutamate neurons (Figure 1), their activation may lead to either an increase or a decrease in glutamate neurotransmission depending on which subpopulations of 5-HT1A receptors are activated.

Figure 1 A schematic diagram of the hypothesized modulatory role of 5-HT receptors on glutamatergic neurotransmission. A glutamatergic pyramidal neuron and several GABA interneurons expressing the 5-HT3, 5-HT1A, 5-HT7, and 5-HT1B receptors on either dendrites or axon terminals are shown. The multimodal compounds vortioxetine and vilazodone and their possible sites of action are also shown. Note that 5-HT1A, 5-HT1B, and 5-HT7 receptors may be localized on different neuronal populations. Symbols used: VLA, vilazodone; VOR, vortioxetine.
Based on the above interaction between effects mediated through the 5-HT1A receptor and glutamatergic neurons, agonists of the 5-HT1A receptor are predicted to have a memory-modulating role, and this has been demonstrated in various preclinical studies.Reference Newman-Tancredi, Martel and Assie 75 – Reference Meneses and Hong 77 The 5-HT1A receptor full agonist flesinoxan impairs working memory in a delayed conditional discrimination task in normal rats.Reference Herremans, Hijzen, Olivier and Slangen 78 Mixed results have been shown in a passive avoidance test in mice, in which pretreatment with flesinoxan either decreased or increased memory function, depending on when it was administered.Reference Tsuji, Takeda and Matsumiya 79 In contrast, a memory-enhancing profile was consistently observed with 5-HT1A agonism in animals with learning and memory deficits. For example, the 5-HT1A receptor agonist 8-OH-DPAT reversed learning deficits induced by scopolamine and MK-801 in an autoshaping learning task.Reference Meneses and Hong 77 Interestingly, a postsynaptic-selective 5-HT1A receptor agonist F15599 was reported to improve working and reference memory in rats with phencyclidine-induced memory deficits.Reference Depoortere, Auclair and Bardin 76 , Reference Horiguchi and Meltzer 80 This seems consistent with the glutamatergic modulatory role of postsynaptic 5-HT1A heteroreceptors. Last, 5-HT1A receptor agonists, such as tandospirone, seem also to be able to alleviate the memory deficits induced by subchronic phencyclidine treatment.Reference Horiguchi and Meltzer 80
Thus, based on the localization and function of 5-HT1A heteroreceptors, 5-HT1A receptor stimulation has the potential to enhance or suppress glutamatergic neurotransmission, and thus may also have biphasic effects on mood or cognitive function.
5-HT1B receptors
Like the 5-HT1A receptors, 5-HT1B receptors are distributed as autoreceptors or heteroreceptors throughout the brain, in areas such as the ventral pallidum, globus pallidus, substantia nigra, dorsal subiculum cerebral cortex, and the hippocampus.Reference Sari 81 Unlike the 5-HT1A autoreceptors, which are localized in somatodendritic regions of 5-HT neurons, 5-HT1B receptors are localized either presynaptically at nerve terminals or postsynaptically on dendrites.Reference Sari 81 – Reference Peddie, Davies, Colyer, Stewart and Rodriguez 83 Postsynaptic 5-HT1B receptors are co-localized with NMDA or AMPA receptors on dentrites, and are thus well-positioned to modulate glutamate transmission.Reference Peddie, Davies, Colyer, Stewart and Rodriguez 82 , Reference Peddie, Davies, Colyer, Stewart and Rodriguez 83 Recently, Cai etalReference Cai, Kallarackal and Kvarta 84 demonstrated that 5-HT1B receptor agonism increases hippocampal excitatory field potentials through a CaM kinase-dependent pathway. In the dorsal subiculum, however, 5-HT1B receptors are localized on CA1 pyramidal axon terminals as inhibitory heteroceptors,Reference Ait, Segu, Naili and Buhot 85 and activation of these receptors attenuates glutamate transmission in the hippocampus due to its negative coupling to adenylate cyclase.Reference Boeijinga and Boddeke 86 – Reference Stepien, Chalimoniuk and Strosznajder 88
The 5-HT1B receptor has been implicated in the pathophysiology and treatment of depression.Reference Svenningsson, Chergui and Rachleff 89 , Reference Tatarczynska, Klodzinska, Stachowicz and Chojnacka-Wojcik 90 It has been shown that the 5-HT1B receptor agonist CP-94253 can modulate 5-HT synthesis in the Flinders Sensitive Line rat, an animal model of depression.Reference Skelin, Kovacevic, Sato and Diksic 91 In intracerebral microdialysis studies, stimulation of 5-HT1B receptors by RU 24969 potentiated the antidepressant-like effects of SSRIs and imipramine.Reference Redrobe, MacSweeney and Bourin 92 Additionally, 5-HT1B receptor stimulation with the selective agonist CP-94253 in mice displayed an antidepressant-like profile in the forced swim test.Reference Tatarczynska, Klodzinska, Stachowicz and Chojnacka-Wojcik 90
The 5-HT1B receptor may modulate learning and memory through a glutamatergic mechanism. Intrahippocampal microinjection of the 5-HT1B receptor agonist CP-93129 impairs spatial learning performance in the radial maze task.Reference Buhot, Patra and Naili 93 On the other hand, the 5-HT1B receptor antagonist SB-224289 enhanced memory consolidation during learning in an associative autoshaping learning task, and reversed the cognitive deficits induced by either the cholinergic inhibitor scopolamine or the NMDA receptor antagonist MK-801.Reference Meneses 94 In an aversive contextual learning task in mice, the 5-HT1B receptor antagonist NAS-181 dose-dependently improved passive avoidance retention.Reference Eriksson, Madjid and Elvander-Tottie 95
Thus, 5-HT1B receptors may be able to positively or negatively modulate glutamate transmission and may be linked to the pathophysiology of depression. Due to the somewhat contrasting antidepressant-like properties of 5-HT1B receptor agonism and memory deficit-reducing effect of 5-HT1B receptor antagonism, a balance of stimulation versus blockade of this receptor may be needed. Based on this idea, a partial agonist for the 5-HT1B receptor may be a reasonable approach, although at the time of writing, the authors are not aware of any empirical investigations of the effects of 5-HT1B partial agonism on mood and cognitive function.
5-HT3 receptors
Among 5-HT receptors, the 5-HT3 receptor is the only known excitatory ion channel, and is expressed throughout the brain, including the following regions: (1) hippocampus; (2) amygdala; and (3) entorhinal, frontal, and cingulate cortices.Reference Thompson and Lummis 96 Immunohistochemical studies show that 5-HT3 receptors are localized in postsynaptic dendrites, especially of GABAergic interneurons in cortical and hippocampal regions.Reference Puig, Santana, Celada, Mengod and Artigas 97 , Reference Morales and Bloom 98 These receptors function as a mechanism of 5-HT-mediated excitation of GABA neurons.Reference Puig, Santana, Celada, Mengod and Artigas 97 In freely moving rats, the 5-HT3 receptor antagonist ondansetron significantly suppressed the firing rate of CA1 hippocampal GABAergic interneurons and concomitantly increased the firing rate of glutamatergic pyramidal cells by disinhibition.Reference Reznic and Staubli 99 Consistent with the above, activation of 5-HT3 receptors can suppress both the spontaneous firing and NMDA-evoked responses of the pyramidal neurons in the rat medial prefrontal cortex.Reference Ashby, Minabe, Edwards and Wang 100 , Reference Liang, Arvanov and Wang 101 Thus, 5-HT3 receptor antagonism enhances glutamate transmission by reducing GABA-mediated inhibition, as illustrated in Figure 1.
This mechanism may explain previous reports that 5-HT3 receptor antagonism by ondansetron enhances long-term potentiation (LTP) and hippocampal and cortical theta rhythms.Reference Staubli and Xu 102 , Reference Sanchez, Robichaud, Pehrson and Leiser 103 Likewise, 5-HT3 receptor antagonists also improve memoryReference Staubli and Xu 102 , Reference Pitsikas and Borsini 104 – Reference Fontana, Daniels, Henderson, Eglen and Wong 107 in preclinical studies. For example, the 5-HT3 receptor antagonist itasetron showed memory-enhancing effects in a multiple-choice avoidance behavioral task,Reference Pitsikas and Borsini 104 and ondansetron blocks scopolamine-induced deficits in learning.Reference Carey, Costall and Domeney 108 In addition to the previously mentioned effects on cognition, 5-HT3 receptor antagonists have antidepressant-like effects. The antagonists such as zacopride and ondansetron reversed helpless behavior in rats.Reference Martin, Gozlan and Puech 109 Newer antagonists also show antidepressant-like activities in the forced swim test and in olfactory bulbectomized rats.Reference Mahesh, Bhatt and Devadoss 110 5-HT3 receptor antagonists also augment the effects of SSRIs.Reference Kos, Popik and Pietraszek 111 , Reference Mørk, Pehrson and Tottrup 112
In conclusion, 5-HT3 receptor antagonism shows antidepressant-like activity and increased cognitive function in preclinical studies, possibly through facilitation of glutamate neurotransmission by reducing the activity of inhibitory GABA neurons.
5-HT7 receptors
The 5-HT7 receptor is a G-protein-coupled receptor (GPCR) with positive coupling to adenylate cyclase, and is highly expressed in the brain, including the thalamus, hypothalamus, hippocampus, and cortex.Reference Hedlund and Sutcliffe 113 In midbrain slices of rat brain containing the dorsal and median raphe nuclei, the mixed 5-HT receptor agonist 5-carboxamido-tryptamine inhibited glutamate release, and this was reversed by the 5-HT7 receptor antagonist SB-258719.Reference Harsing 114 Thus, 5-HT7 receptors in the axon terminals of the glutamatergic cortico-raphe neurons may serve as heteroreceptors that inhibit glutamate release.Reference Harsing 114 , Reference Duncan and Congleton 115 The 5-HT7 receptor is also expressed on the cell bodies of pyramidal neurons.Reference Bickmeyer, Heine, Manzke and Richter 116 In normal animals, activation of the 5-HT7 receptor leads to increased firing of glutamatergic neurons in the cortexReference Fan, Zhang and Liu 117 and hippocampus.Reference Tokarski, Zahorodna, Bobula and Hess 118 However, these effects on glutamatergic neurotransmission may be accompanied by increased inhibitory GABAergic transmission, likely due to expression in both pyramidal neurons and GABAergic interneurons. These concomitant effects were demonstrated in the hippocampus with an increase in the frequencies of both spontaneous inhibitory postsynaptic currents recorded in pyramidal neurons and spontaneous excitatory postsynaptic currents recorded in interneurons.Reference Tokarski, Kusek and Hess 119 Based on these data, 5-HT7 receptor activation has mixed effects on glutamatergic neurotransmission, but the overall effect in normal rodents appears to be excitatory.Reference Fan, Zhang and Liu 117 Importantly, this relationship may be altered in disease states, as 5-HT7 receptor activation in 6-hydroxydopamine-lesioned animals led to a net inhibition, rather than excitation, of pyramidal cell firing in the same study.Reference Fan, Zhang and Liu 117 Based on these results, 5-HT7 receptor antagonism may result in either increases or decreases in glutamatergic neurotransmission within the context of depression.
Although the effects of 5-HT7 receptor modulation on glutamatergic neurotransmission are currently somewhat unclear, clear antidepressant-like activities of 5-HT7 antagonism have been reported in a number of preclinical studies. Treatment with the 5-HT7 receptor antagonist SB-269970 reduced immobility in the forced swim and tail suspension tests, and there was a further synergistic effect on extracellular 5-HT release in the frontal cortex when SB-269970 was combined with the SSRI citalopram.Reference Bonaventure, Kelly and Aluisio 120 Therefore, the results from preclinical studies suggest that 5-HT7 receptor antagonism might be a novel strategy for treating depression.Reference Hedlund 121 , Reference Stahl 122
Additionally, memory-enhancing effects of 5-HT7 antagonists have been shown in preclinical modelsReference Meneses 123 – Reference Waters, Stean and Hammond 126 and have been reviewed elsewhere.Reference Stahl 122 In cases where learning or memory was disrupted by NMDA antagonists such as phencyclidine or MK-801, 5-HT7 receptor antagonism consistently improved performance.Reference Meneses 123 – Reference Horiguchi, Huang and Meltzer 125 , Reference Bonaventure, Aluisio and Shoblock 127 Interestingly, combined 5-HT7 receptor antagonism and SERT inhibition produced a synergistic effect in a preclinical test of executive function.Reference Nikiforuk 128
These data support a modulatory role of 5-HT7 receptors on glutamate transmission as mentioned above. 5-HT7 receptor antagonism might be beneficial to cognitive function and antidepressant activity.
Multimodal Antidepressants
There are currently two multimodal compounds with clinically documented antidepressant activity: vilazodone, which is approved for clinical use in the U.S., and vortioxetine, which is undergoing regulatory review. Given the complexity of the serotonergic modulation of glutamate, it is not possible to predict the net effect that multimodal serotonergic compounds will have on glutamate neurotransmission. Thus, the need for empirical data on the effects of these compounds on glutamate neurotransmission is paramount.
Vilazodone is a recently approved antidepressant with high affinities for the SERT (IC50 0.5 nM) and 5-HT1A receptor (EC50 0.2 nM)Reference Heinrich, Bottcher and Gericke 129 , Reference Page, Cryan and Sullivan 130 (Table 2). Vilazodone is a partial agonist at the 5-HT1A receptor, but with a relatively high intrinsic activity—69% of the magnitude of the full 5-HT1A receptor agonist 8-OH-DPAT.Reference Page, Cryan and Sullivan 130 In preclinical studies, vilazodone seems to outperform the SSRIs paroxetine and fluoxetine, as measured by 5-HT release and ultrasonic vocalization. However, the fact that antidepressant-like effects are observed at moderate but not higher doses in the rat and mouse forced swim test may suggest that its 5-HT1A receptor partial agonism may inhibit the expression of rodent antidepressant-like behaviors.Reference Page, Cryan and Sullivan 130 , Reference De Paulis 131 Vilazodone's potential to interact with glutamate neurotransmission is illustrated in Figure 1. The antidepressant efficacy of vilazodone was seen only in some of the clinical trials, partly due to the need to balance the higher dose (40 mg) needed versus the high rate of gastrointestinal side effect, and thus its efficacy and safety profiles in comparison to current antidepressants require further clinical evaluation.Reference Guay 132 , Reference Wang, Han, Lee, Patkar and Pae 133
Table 2 Clinical compounds with serotonin (5-HT) transporter (SERT) inhibition plus activity at one or more 5-HT receptors linked to glutamatergic modulation
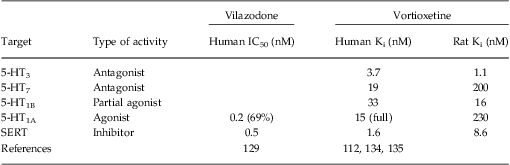
The in vitro pharmacological activities were from either binding or functional measurements. Numbers in parentheses denote agonist efficacy.
Vortioxetine is an investigational multimodal antidepressant that acts as a 5-HT3, 5-HT7, and 5-HT1D receptor antagonist; 5-HT1B receptor partial agonist; 5-HT1A receptor agonist; and SERT inhibitor in vitroReference Mørk, Pehrson and Tottrup 112 , Reference Bang-Andersen, Ruhland and Jørgensen 134 , Reference Westrich, Pehrson and Zhong 135 (Table 2). Its pharmacological profile indicates that vortioxetine has the potential to modulate glutamate transmission through all of the four 5-HT receptor pathways discussed above (Figure 1). Multiple reports of preclinical studies have shown the antidepressant-like activities of vortioxetine.Reference Mørk, Pehrson and Tottrup 112 , Reference Bang-Andersen, Ruhland and Jørgensen 134 – Reference Li, Pehrson, Budac, Sanchez and Gulinello 138 Further, in clinical studies, its efficacy as an antidepressant has been demonstrated in several studies to date,Reference Alvarez, Perez, Dragheim, Loft and Artigas 139 – Reference Baldwin, Hansen and Florea 145 although statistically significant separation from placebo has not been observed in every clinical trial.Reference Mahableshwarkar, Jacobsen and Chen 146 , Reference Jain, Mahableshwarkar, Jacobsen, Chen and Thase 147 Recently, it was reported that vortioxetine enhanced time-dependent contextual fear memory and object recognition memory in rats.Reference Mørk, Montezinho and Miller 148 Additionally, 5-HT depletion-induced memory deficits were dose-dependently reversed by vortioxetine treatment,Reference Pehrson, Gaarn du Jardin Nielsen, Jensen and Sanchez 106 while escitalopram and duloxetine were inactive. These data strongly suggest that the receptor activities of vortioxetine contribute to its cognition-improving properties in rats.Reference Pehrson, Gaarn du Jardin Nielsen, Jensen and Sanchez 106 In further support of the relevance of the receptor mechanism, this study reported improved memory performance in rats by a selective 5-HT1A receptor agonist and a 5-HT3 receptor antagonist.Reference Pehrson, Gaarn du Jardin Nielsen, Jensen and Sanchez 106 Furthermore, a recent clinical study in elderly depressed patients showed a beneficial effect of vortioxetine compared to placebo in cognitive tests of processing speed, verbal learning, and memory.Reference Katona, Hansen and Olsen 140 It should be noted that vortioxetine has a 10-fold lower in vitro affinity for rat 5-HT7 (Ki = 200 nM) compared with human 5-HT7 receptors (Ki = 19 nM), and a ∼15-fold lower affinity at rat 5-HT1A (Ki = 230 nM) compared with human 5-HT1A receptors (Ki = 15 nM)Reference Mørk, Pehrson and Tottrup 112 (Table 2). Thus, the contribution of the 5-HT7 and 5-HT1A receptors in the clinic may be underestimated by evaluation of preclinical models. Based on the current preclinical understanding of the mechanisms and the preclinical and clinical results, we hypothesize that vortioxetine's multimodal profile including 5-HT3 and 5-HT7 antagonism, 5-HT1B partial agonism, and 5-HT1A agonism could result in enhanced glutamate transmission and contribute to its antidepressant and cognitive enhancing properties (Figure 1). However, the way in which vortioxetine modulates glutamate transmission remains to be empirically determined.
Conclusions
Pharmacological treatments for major depressive disorder have evolved from monoamine-based therapies to integration of glutamatergic mechanisms. Data from current clinical and preclinical compounds targeting NMDA, AMPA, and mGluR receptors and glutamate transport present new opportunities for the treatment of depression. The serotonergic system can modulate glutamate transmission through 5-HT3, 5-HT1A, 5-HT7, and 5-HT1B receptors. These 5-HT receptor targets present opportunities for integrating glutamatergic modulation into monoamine-based therapies, without the direct use of glutamatergic compounds. The multimodal compounds vilazodone and vortioxetine are examples of this approach with diverse mechanisms, to indirectly modulate glutamate transmission by respectively targeting the 5-HT1A receptor, or 5-HT3, 5-HT1A, 5-HT7, and 5-HT1B receptors along with the SERT. Clinical results with these multimodal compounds will provide valuable insights into whether exploiting serotonergic modulation of glutamate transmission is an effective strategy in treating depression.
Disclosures
The work by both authors was performed as full-time employees of Lundbeck at the time of the study. Vortioxetine is currently under development by H. Lundbeck A/S and the Takeda Pharmaceutical Company, Ltd.







