Introduction
Vulture species across the globe face numerous threats to their survival. Many of these challenges are the result of human persecution, development, and unintentional poisoning (Green et al. Reference Green, Newton, Shultz, Cunningham, Gilbert, Pain and Prakash2004, Oaks et al. Reference Oaks, Gilbert, Virani, Watson, Meteyer, Rideout, Shivaprasad, Ahmed, Chaudhry, Arshad, Mahmood, Ali and Khan2004, Hernández Reference Hernández and Margalida2008, Margalida Reference Margalida, Heredia, Razin and Hernandez2008, Hernández and Margalida Reference Hernández and Margalida2009). Catastrophic declines of vultures belonging to the genus Gyps have been documented throughout India and Pakistan, as well as more widely across the Indian sub-continent, in recent years (Prakash Reference Prakash1999, Prakash et al. Reference Prakash, Pain, Cunningham, Donald, Prakash, Verma, Gargi, Sivakumar and Rahmani2003, Gilbert et al. Reference Gilbert, Virani, Watson, Oaks, Benson, Khan, Ahmed, Chaudhry, Arshad, Mahmood and Shah2002, Oaks et al. Reference Oaks, Gilbert, Virani, Watson, Meteyer, Rideout, Shivaprasad, Ahmed, Chaudhry, Arshad, Mahmood, Ali and Khan2004, Hla et al. in press, Chaudhary et al. in press). The first early warning sign was detected in India’s Keoladeo National Park in the 1990s, when White-backed Vultures Gyps bengalensis, then one of the most common raptors on the Indian subcontinent, began a massive decline (Prakash Reference Prakash1999). Since then, catastrophic declines, also involving the Indian Gyps indicus, formerly G. indicus indicus and Slender-billed Vulture Gyps tenuirostris, formerly G. indicus tenuirostris, have been reported across the subcontinent (Prakash et al. Reference Prakash, Pain, Cunningham, Donald, Prakash, Verma, Gargi, Sivakumar and Rahmani2003). Not surprisingly, these species are now listed as ‘Critically Endangered’ (BirdLife International 2009) and a flurry of research activity has attempted to identify the underlying cause of these population crashes.
These crashes have been largely attributed to poisoning by diclofenac, a non-steroidal anti-inflammatory drug used widely to treat sick and arthritic livestock (Green et al. Reference Green, Newton, Shultz, Cunningham, Gilbert, Pain and Prakash2004, Reference Green, Taggart, Das, Pain, Kumar, Cunningham and Cuthbert2006, Oaks et al. Reference Oaks, Gilbert, Virani, Watson, Meteyer, Rideout, Shivaprasad, Ahmed, Chaudhry, Arshad, Mahmood, Ali and Khan2004, Shultz et al. Reference Shultz, Baral, Charman, Cunningham, Das, Ghalsasi, Goudar, Green, Jones, Nighot, Pain and Prakash2004, Taggart et al. Reference Taggart, Cuthbert, Das, Sashikumar, Pain and Green2006, Taggart et al. Reference Taggart, Senacha, Green, Jhala, Raghavan, Rahmani, Cuthbert, Pain and Meharg2007, Das et al. 2011). Vultures that feed on diclofenac-laced carcasses can suffer renal failure and visceral gout (Oaks et al., Reference Oaks, Gilbert, Virani, Watson, Meteyer, Rideout, Shivaprasad, Ahmed, Chaudhry, Arshad, Mahmood, Ali and Khan2004, Shultz et al. Reference Shultz, Baral, Charman, Cunningham, Das, Ghalsasi, Goudar, Green, Jones, Nighot, Pain and Prakash2004, Swan et al. Reference Swan, Cuthbert, Quevedo, Green, Pain, Bartels, Cunningham, Duncan, Meharg, Oaks, Parry-Jones, Shultz, Taggart, Verdoorn and Wolter2006), which caused high mortality and led to dramatic population declines (34–95%) of White-backed Vultures in Pakistan between 2000 and 2003 (Oaks et al. Reference Oaks, Gilbert, Virani, Watson, Meteyer, Rideout, Shivaprasad, Ahmed, Chaudhry, Arshad, Mahmood, Ali and Khan2004). A follow-up study purported to show that diclofenac concentrations in livestock carcasses were sufficient to explain documented declines of all three above mentioned Gyps species across the Indian subcontinent (Green et al. Reference Green, Taggart, Das, Pain, Kumar, Cunningham and Cuthbert2006). The evidence that diclofenac played a key causal role in the vulture die-off is compelling.
However, the potential role of climate in connection with vulture declines has not been thoroughly explored thus far. There is a growing body of evidence that climatic factors including El Niño Southern Oscillation (ENSO) events can have dramatic negative effects on populations (Chhangani Reference Chhangani2004, Reference Chhangani2005, Chhangani and Mohnot Reference Chhangani and Mohnot2004, Waite Reference Waite, Campbell, Robbins and Chhangani2007a,Reference Waite, Chhangani, Campbell, Rajpurohit and Monotb, Chhangani and Sivaperuman Reference Chhangani, Sivaperuman, Sivaperuman, Baqri, Ramaswamy and Naseema2009). In arid regions, ENSO alternates between El Niño events leading to rainfall and La Niña events leading to drought. By suppressing plant growth and recruitment, drought can have bottom-up trophic effects (Holmgren and Scheffer Reference Holmgren and Scheffer2001), leading to vertebrate population crashes (Harrison Reference Harrison2000, Cleary et al. Reference Cleary, Fauvelot, Genner, Menken and Mooers2006). In the case of the recent crash of Gyps spp., the role of climate seems worth considering because the crash coincided with the major ENSO-induced drought of 2000, which apparently caused a simultaneous decline of the mammal community in a nearby wildlife sanctuary in the Aravalli Hills (Waite et al. Reference Waite, Campbell, Robbins and Chhangani2007a). Given the widespread use of diclofenac among livestock (Green et al. Reference Green, Newton, Shultz, Cunningham, Gilbert, Pain and Prakash2004) it seems plausible that the coincidental crash of vultures could have been exacerbated by the same ENSO-induced drought.
Here, we examine the impact of ENSO on population dynamics of the Indian Vulture at 11 village sites scattered throughout western Rajasthan, India. We ask whether ENSO-induced drought synchronised population dynamics across the region. We also model village-specific vulture population growth rates in order to discover which variables contributed to respective trends in vulture populations over time.
Methods
Study area
The study area was situated within 175 km of Jodhpur (26o18′N, 73o08′E), a major city (population ∼1.5 million) in western Rajasthan (Figure 1), at the eastern edge of the Great Indian (Thar) Desert. The region is covered with open scrub forest. Dominant tree and shrub species include: Acacia senegal, A. nilotica, Euphorbia caducifolia, Anogeissus pendula, Mytenus emarginata, Grewia tenax, Ziziphus nummularia, Prosopis cineraria, Capparis decidua, Tecomella undulata and the exotic invasive Prosopis juliflora. Temperatures can reach highs of ∼ 48oC in May and June, and lows of ∼ 1oC during winter. Annual rainfall averages 360 mm, with 90% occurring during the monsoon season (July–September). Monsoon failure can lead to hydrological and vegetative drought.
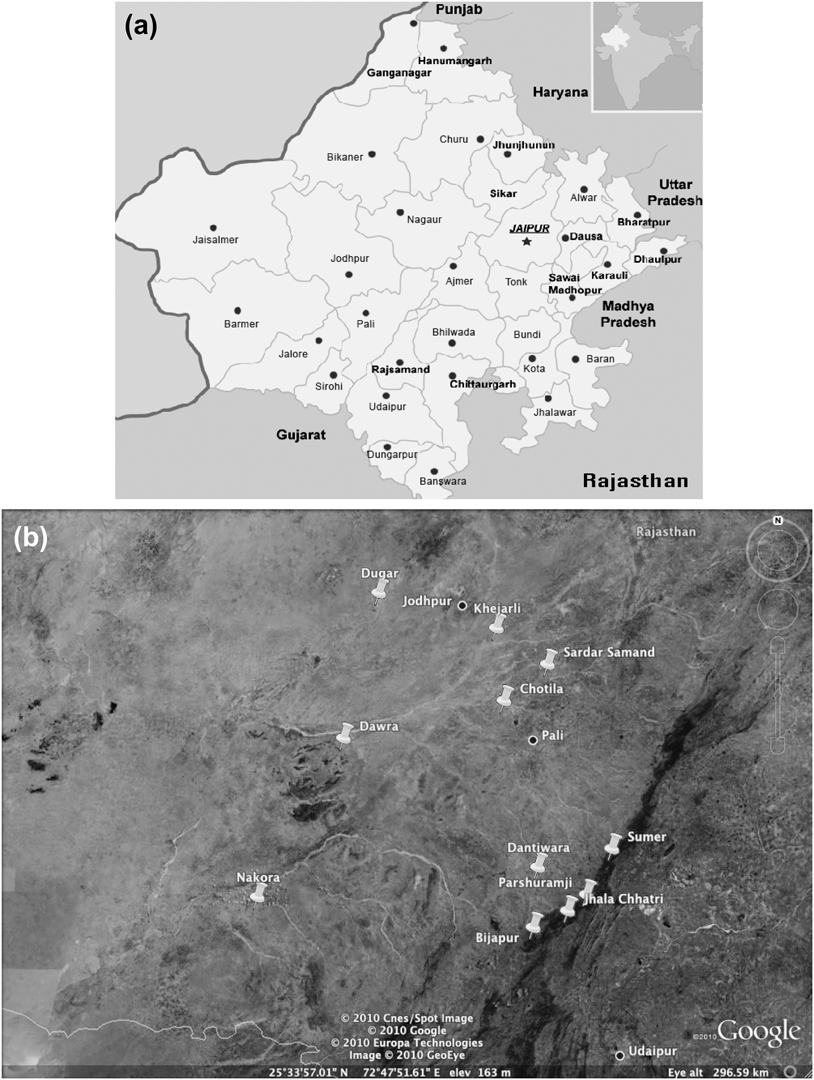
Figure 1. a) Map of Rajasthan. b) Map of village locations in relation to the major cities Jodhpur and Pali. Push pin icons mark village sites.
Villages are composed of a central housing complex (multiple homes all within 1 km of each other) and surrounding agricultural and community lands. Boundaries between villages are established via landmarks on individual or village lands.
Study species
The Indian Vulture is a large (5.5–6.3 kg) scavenging raptor found almost throughout the Indian subcontinent (for a detailed description see Ferguson-Lees et al. Reference Ferguson-Lees, Christie, Franklin, Philip and Mead2001, BirdLife International 2009). The Indian Vulture feeds almost exclusively on carrion, mostly livestock carcasses. It typically nests in colonies on cliffs or crags, but also nests in trees where rocky habitat is unavailable (Chhangani and Monot Reference Chhangani and Mohnot2004, Chhangani Reference Chhangani2005, Chhangani and Sivaperuman Reference Chhangani, Sivaperuman, Sivaperuman, Baqri, Ramaswamy and Naseema2009). Nesting begins in November and lasts until May, with successful pairs raising one offspring per breeding season.
The Indian Vulture was recently granted species status, when two subspecies of the Long-billed Vulture were split into Indian and Slender-billed Vultures (Rasmussen and Parry Reference Rasmussen and Parry2001). Despite morphological similarities, and some overlap in geographic range between these species, we can state confidently that we collected data on Indian Vultures only. None of our vultures showed the diagnostic field marks of Slender-billed Vulture (slender bill and dark brown plumage on body and head; Ferguson-Lees et al. Reference Ferguson-Lees, Christie, Franklin, Philip and Mead2001) and none of the local populations we monitored occurred in the area of known overlap in geographic ranges (IUCN 2007).
Field methods
Monitoring of vultures in and around 11 villages within 175 km of the city of Jodhpur (Figure 1) began in winter 1996/1997. Annual counts were conducted until 2004/2005. Vultures were counted at known nesting sites within each village in trees and rock formations. Local villagers were often consulted as to where they had observed vulture nesting sites. Observations were also made from vehicles during road transects and opportunistically during periodic visits to villages in the region. Adult breeding pairs were counted and recorded as two individuals along with offspring in each respective nest. The known local breeding populations included in our analysis were surveyed extensively during each nesting season.
These data provide an index of local abundance of Indian Vultures during the nesting season. Because vultures were not individually identifiable, and because our monitoring efforts were periodic rather than continuous, we do not know the precise relationship between observed and actual abundance. Therefore, our analysis is based on the assumption that the index of abundance is proportional to actual abundance and that this proportionality holds steady across years and locations.
Climate data
We used an El Niño Southern Oscillation (ENSO) index as a predictor of Indian Vulture population dynamics. ENSO is the most important oceanic-atmospheric driver of year-to-year variability in global climate. We used the Multivariate ENSO Index (MEI) (http://www.cdc.noaa.gov/people/klaus.wolter/MEI/mei.html#ref_wt1), which is based on six variables observed over the tropical Pacific Ocean: sea-level pressure, zonal and meridional components of surface wind, sea surface temperature, surface air temperature, and total fractional cloudiness. MEI is computed for sliding bimonthly periods, and standardised with respect to season and the reference interval 1950–1993. We used mean MEI values during winter, December–February. Negative values represent the cold ENSO phase (La Niña), while positive values represent the warm ENSO phase (El Niño).
Data analysis
All the time-series described below were analysed using SPSS (2007). All statistical tests were two-tailed.
Cross-correlation, ‘Leave-one-out’ analysis of synchrony
To evaluate whether ENSO might have synchronised Indian Vulture population dynamics across the region, we began by cross-correlating (lag = 0) the time series of the local populations. We did so after first transforming (natural logarithm) and differing (1 year) the data, thereby converting the time series of raw count data into time series of annual population growth rates (i.e., ln[Nt]-ln[Nt -1]) (N = number of vultures). We asked whether local population growth rate from year to year co-varied across the region, as expected if climate had an overwhelming influence.
Finding considerable evidence for synchronous population dynamics, we next evaluated whether this synchrony might have been driven by two La Niña events, one ending in 1997 and another spanning 1999 (Figure 2). We reasoned that these events might have triggered downturns in local populations throughout the region and so might have had a major synchronising influence. To evaluate this possibility, we repeated the cross-correlation analysis described above while leaving out (without replacement) the interannual growth for each year. We reasoned that leaving out growth rate estimates for years coinciding with drought would yield a suppressed mean correlation coefficient. Any such cases would indicate that drought contributed to the overall synchrony in population dynamics across the region.

Figure 2. Time series of Multivariate ENSO Index (MEI). Positive MEI values indicate El Niño events and negative values indicate La Niña events.
Time series analysis of ENSO impact
We used Auto Regressive Integrated Moving Average (ARIMA) models (Carroll and Pearson Reference Carroll and Pearson1999, Becerra-Muñoz et al. Reference Becerra-Muñoz, Buelna-Osben and Catalán-Romero2003) to evaluate more directly whether ENSO influenced Indian Vulture population dynamics. Specifically, we asked whether annual population growth rate could be adequately explained by MEI during year t alone or together with MEI during year t-1. We used first-order models (i.e. with autoregressive lag term for 1 year [AR1]). Parameters were set as follows: P = 1 (lag of 1 year), d = 1 (time series differenced once to make it stationary, following natural logarithm transformation), and q = 0 (order of moving average set to zero).
We used the Akaike Information Criterion (AIC) (Bozdogan Reference Bozdogan1987) for model selection to evaluate whether the dynamics of each local population were best described by AR1 alone, by AR1 and MEIt, or by AR1, MEIt and MEIt -1. Best models were identified as those with minimal AIC values. We used stationary r 2as the goodness-of-fit measure.
Time series analysis for individual villages
We first determined whether the best AIC-based ARIMA model for each village’s local population included MEIt or MEIt -1. If the best model did not include either metric for ENSO then we assumed the local population was somewhat buffered by the negative effects of drought. We next evaluated whether each model including one or both of these MEI predictors had especially poor fit for this local population. Finally, we asked whether each local population’s time series showed low autocorrelation (transformation: natural logarithm; differencing: 1 year), particularly when MEI predictors were included. We thus reasoned that Indian Vulture population dynamics in any village might have been largely independent of MEI and representative of an underlying white noise process.
Results
ENSO and synchronous population dynamics throughout the region
The MEI time series (Figure 2) includes two La Niña events, as shown by the prolonged series of negative MEI values extending into 1997 and the subsequent series spanning 1999. Such events can lead to monsoon failure. The major event spanning 1999 caused consecutive monsoon failures leading to a severe vegetative drought throughout Rajasthan in 2000. Our time series of Indian Vulture population growth rates suggests that both of these La Niña events had a latent impact. Table 2reveals that all 11 local populations shrank from 1997 to 1998 and again from 1999 to 2000. These universal downturns suggest that ENSO-induced drought might have synchronised population dynamics across the region.
Table 1. Vulture counts from each of the 11 villages, 1996–2005.

Table 2. Annual population growth rates of Indian Vultures in each surveyed village, 1996–2005.

Table 3 confirms a high degree of region-wide synchrony. The time series of population growth rates were universally positively correlated across the 55 pairwise combinations of local populations, with 18 of these correlations reaching nominal significance (i.e. P < 0.05, unadjusted for multiplicity). Our leave-one-out analysis revealed three inter-annual growth rates that most strongly influenced this synchrony. Specifically, leaving out the growth rates for these inter-annual intervals (1997–1998, 1999–2000 and 1996–1997) suppressed the mean correlation coefficient (0.309, 0.534, and 0.519, respectively) below the original value of 0.564 (i.e. the mean of values in Table 2). The strong region-wide synchrony was driven by the universal downturns in 1998 and 2000, following the two La Niña events (and by the universal upturn from 1996 to 1997) (Table 2).
Table 3. Cross-correlation matrix for time series of annual population growth rate for 11 local populations (named after villages) of the Indian Vulture. All 55 pairwise combinations of time series were positively correlated, indicating region-wide synchrony. Bold face indicates nominal significance (P < 0.05, unadjusted for multiplicity).

Time series analysis using ARIMA models further suggests that ENSO influenced population dynamics. Table 4 shows the best AIC-based model included at least one MEI predictor for all eleven villages in the study. Table 5shows relatively good fit for models including one or both MEI predictors.
Table 4. Local populations of Indian Vulture ordered from highest to lowest mean population growth rate across the time series, 1996–2005. Also shown are predictor variables included in the best AIC-based ARIMA models.

Table 5. Goodness-of-fit as measured by stationary r 2 for first-order ARIMA models. Villages (local breeding populations of Indian Vultures) ordered from best to worst for the model with predictors AR1+MEIt.

Individual village variation in vulture populations under drought conditions
We report several lines of preliminary evidence that Indian Vultures in several villages were buffered against ENSO impacts. Dugar, Khejarli, and Chotila’s respective local populations had the most favourable population growth rates, with Dugar having a net population change of zero (Table 4). Second, for all three of these villages’ local populations, the fit of the ARIMA model was the poorest of all when one or both of the MEI predictors were included (Table 5). Lastly, autocorrelation analysis revealed that annual population growth rates for each of the three villages’ respective populations were serially independent (Table 6), as expected for an underlying white noise process. Overall, the Indian Vulture populations in Khejarli, Dawra, and Sumer appeared to be less influenced by the vagaries of ENSO.
Table 6. Results of autocorrelation analysis on time series of Indian Vulture count data (transformation: natural logarithm; differencing: 1 year). Smaller Box-Ljung statistic values indicate stronger support for an underlying white noise process. Villages (local breeding populations of Indian Vultures) ordered based on ascending values of this statistic. Local breeding populations of Indian Vulture in villages nearer the bottom of the list were apparently less influenced by ENSO.

a Two-tailed, based on chi-square distribution (df = 1).
Discussion
We offer several insights regarding population dynamics of the Indian Vulture, a Critically Endangered species of the Indian subcontinent. Population dynamics were synchronous across western Rajasthan, as if a large-scale abiotic driver overwhelmed intrinsic regulatory factors. Our analysis suggests ENSO was the major synchronising factor. In part, a major La Niña-induced drought in 2000 apparently triggered a universal downturn in local populations throughout the region. Despite this widespread synchrony, several local populations appeared to be less influenced by ENSO, providing the impetus to further investigate other possible influences on Indian vultures nesting in these villages.
ENSO and Indian Vulture population dynamics
Our results indicate that ENSO events synchronised Indian Vulture population dynamics throughout western Rajasthan. We suggest that the major La Nina event spanning 1999 might have played a role in triggering the downturn in Indian Vultures throughout western Rajasthan (Chhangani Reference Chhangani2005). Until now, diclofenac has been implicated as the major, perhaps only, cause of the widespread decline of this species (Green et al. Reference Green, Newton, Shultz, Cunningham, Gilbert, Pain and Prakash2004). Our intent is not to discredit evidence that diclofenac poisoning has played a major causal role or to question IUCN’s diclofenac-related rationale for recently listing the Indian, White-backed, and Slender-billed Vulture as ‘Critically Endangered’. We simply emphasise that the potentially additive or synergistic role of climate should not be ignored, especially in an arid region where ENSO-induced drought is now known to cause catastrophic die-offs of vertebrates, even in a protected area and presumably in the absence of diclofenac poisoning (Waite et al. Reference Waite, Campbell, Robbins and Chhangani2007a). Future attempts to model causes of widespread population declines should begin by incorporating the climate cycle as the primary abiotic driver.
Buffering from drought in villages
While any attempt to understand population dynamics should consider the role of climate, there are several contributing factors, including human activity, that can potentially buffer vertebrate populations against climatic vagaries, even protecting them against catastrophic die-off during drought (Waite et al. Reference Waite, Chhangani, Campbell, Rajpurohit and Monot2007b). In keeping with the growing emphasis on community-based conservation in developing countries (Agrawal and Gibson Reference Agrawal and Gibson1999, Lepp and Holland Reference Lepp and Holland2006, van Eeden et al. Reference van Eeden, van Rensburg, De Wijn and Bothma2006, Sommerville et al. Reference Sommerville, Milner-Gulland, Rahajaharison and Jones2010, Waylen et al. Reference Waylen, Fischer, McGowan, Thirgood and Milner-Gulland2010, López-Arévalo et al. Reference López-Arévalo, Gallina, Landgrave, Martinez-Meyer and Muñoz-Villers2011) we would like to specifically explore the potential impact that humans living in the region may have on Indian Vulture populations. Permanent food and water supply and nesting habitat are factors that are positively associated with sustained vulture populations (Chhangani Reference Chhangani2005, Monadjem and Garcelon Reference Monadjem and Garcelon2008, Mateo-Tomás and Olea Reference Mateo-Tomás and Olea2010). Future research will further investigate village-specific characteristics such as small cliff formations (Dawra village), large temples (Dugar village), and permanent water bodies (Khejarli and Chotila village) and the potential effects on Gyps vulture populations.
Khejarli village is of particular interest however, because of the presence of a unique caste of local people that may have a positive influence on vulture nesting habitat. The Bishnoi people of western Rajasthan could be an exemplar of built-in community-based conservation of vultures and other species of conservation concern. The Bishnoi faith, founded in 1485 by Jambeshwar, is based on twenty-nine principles (“Bish-noi” translates to “twenty-nine”), one of which specifies doing no harm to any living creature (Fisher Reference Fisher1997, Brockmann and Pichler Reference Brockmann and Pichler2004). In particular, they hold sacred a key tree species, Prosopis cineraria (locally known as the khejeri tree) (Brockmann and Pichler Reference Brockmann and Pichler2004). Bishnoi do not cut off branches of khejeri to feed livestock, thereby presumably providing more favourable nesting habitat for vultures (Brockmann and Pichler Reference Brockmann and Pichler2004). Vulture populations in areas with proper nesting habitat may better maintain village-specific birth rates during drought years where adults may emigrate to other areas or experience greater juvenile mortality. Presumably, vultures would avoid areas where khejeri trees are heavily cut and choose to nest in areas where trees are uncut, specifically areas with relatively high Bishnoi populations.
Though more research is on the subject of a “Bishnoi effect” is necessary, we speculate that a portion of the buffering that occurred in Khejarli village is attributable to the protection practices of the Bishnoi people. The Bishnoi have a long history of protecting this tree. In the year 1730, 363 Bishnoi men, women and children sacrificed their lives in protest at Maharaja Abhay Singh felling khereji trees in Khejarli village for renovation of his palace (Brockmann and Pichler Reference Brockmann and Pichler2004). The Bishnoi, who make up 41% of the ~1,200 residents of Khejarli, still actively protect this tree (pers. obs.) and may provide an oasis of suitable, undisturbed nesting habitat for vultures.
Any strong inferences about whether and how Bishnoi activities benefit species of conservation concern cannot, however, be based solely on one species in one village. Future work will expand the geographic scope of this preliminary study and attempt to uncover the underlying basis for how Bishnoi provide a safe haven for endangered wildlife species.
Implications for conservation breeding strategies
In response to the vulture crisis, a captive breeding programme was initiated eight years ago for Indian, White-backed, and Slender-billed Vultures in Pinjore, India (Vulture Rescue 2008). This programme aims to protect remaining populations while giving the country’s ban on diclofenac time to take effect, and then to release captive-reared vultures into the wild. Our results prompt two suggestions for those devising strategies for reintroduction. First, given our evidence for region-wide universal downturns of Indian Vulture populations following La Niña events, we suggest that releases should not be scheduled to coincide with ongoing or imminent La Niña events. Straightforward monitoring of MEI data could help to optimise the timing of release.
Second, while releasing captive-reared vultures in protected areas (national parks or wildlife sanctuaries) seems the obvious preferred approach, it is important that the potentially positive effects of human activity on wildlife are also considered. The miraculous recent recovery of Spot-billed Pelicans Pelecanus philippensis in Cambodia (400% increase in population size in seven years) provides a striking example of successful local community involvement with wildlife conservation (Wildlife Conservation Society 2008). Beyond the speculative benefits provided by their lifestyle, the Bishnoi also opportunistically perform wildlife rescue and rehabilitation, e.g. of injured blackbuck Antilope cervicapra(pers. obs.). Success of recovery efforts succeed depends on the fate of wild populations after reintroduction. Ideally, the ongoing captive breeding efforts will not be squandered by the failure of wild populations in the future. Well-timed release of captive-raised vultures into the wild, combined with local community participation, could improve the prospects for successful recovery of vulture populations.
Conclusions
We found that Indian Vulture population dynamics were synchronised across a broad region of western Rajasthan, apparently by ENSO events. Although ENSO apparently impacted local populations in a parallel way across this region, some local populations were partially buffered from the effects of drought. Factors contributing to drought buffering, including human activity, are currently being investigated. Future attempts to model the effects of putative causes of widespread vulture declines (e.g. by diclofenac poisoning) should incorporate the potentially significant impact of climate. Ongoing research aims to explore whether and how human activity may directly impact the conservation status of the Indian Vulture and other endangered wildlife species, within officially unprotected areas.
Acknowledgements
We thank CSIR, New Delhi for S.R.A. ship; Professor D. Mohna, Head of the Department of Zoology, J.N.V. University, U.S. Fish and Wildlife Service, and The School for Desert Sciences for supporting vulture fieldwork; S. M. Mohnot and L. S. Rajpurohit for valuable guidance; P. Robbins for related collaboration; many support personnel; I. Hamilton, E. Marschall, K. McSweeney, and the Waite Lab Group for helpful feedback; Stauf’s, Soul Sistas, and Seven Main for providing a pleasant workspace; and NSF grant 0351037.












