The opportunity to immunize against diphtheria
Edward Jenner's achievement in describing, in 1798, an essentially safe vaccine against smallpox is universally known. Less well known is the struggle a century later to protect children against diphtheria. That immunization, when eventually in place in the UK, was a turning point in public health policy. It was the start of an expanding centrally funded programme of childhood immunizations against infectious disease.
The diphtheria bacillus had first been seen by Klebs in 1884 and was proved to be the cause of diphtheria by Loeffler in 1885. Loeffler also observed that some healthy children carried the organism. In 1888 Roux and Yersin described the diphtheria exotoxin, then Behring, Fraenkel, Ehrlich and Kitasato in Berlin and Roux and Martin in Paris showed that immune serum could neutralize its effects.
At Christmas 1891, in an atmosphere of Franco-Prussian rivalry, two diphtheritic children in a Berlin hospital were saved by the administration of Behring's immune sheep serum, an outcome since described by one medical historian (referring to Pasteur's successful treatment in 1885 of a boy badly bitten by a rabid dog) as ‘outpasteuring Pasteur’ [Reference Porter1]. Of more lasting significance, though, was the French achievement in raising diphtheria antitoxic serum in horses. Roux's horses yielded serum in greater volume and of higher titre than Behring's sheep, and it was horse antitoxin that gradually came into general use. By 1894 both the French and the German groups had clinical data on series of several hundred children, and had shown that with antitoxin treatment diphtheria mortality fell from over 50% to less than 25%.
The first known use of diphtheria antitoxin in Britain, in 1894, was on a nephew of the physiologist Charles Sherrington [Reference Parish2]: it was a dramatic moment in British bacteriology. Sherrington, having recently learnt how horses were being immunized in Paris, had begun to inject a horse, ‘Tom’, in London (foreshadowing the scaled up production of horse antiserum at the Lister Institute [Reference Chick, Hume and Macfarlane3]). The general practitioner attending Sherrington's sick nephew had told him: ‘You can do what you like with the boy, he will not be alive at teatime’; but Sherrington raced to his nephew's bedside with serum he had just collected from Tom. The child recovered.
At the turn of the century diphtheria was still the cause of one in seven deaths in British children [Reference Forbes4]. Timely administration of antitoxic serum had the potential to lower mortality and protect contacts, but it was some years before the immunization of horses was optimized, and the early diagnosis of diphtheria was not straightforward. Moreover, the serum sometimes induced serum sickness. Diagnostic hesitancy meant that the New York physician William Park could still complain in 1922 that
the combination of the ever present carrier of bacilli and the slowness of people to recognise diphtheria when it develops … permits the occurrence of 50% more diphtheria than we would otherwise have [Reference Park5].
Antitoxin alone was never going to solve the problem of diphtheria; instead an effective and long lasting pre-infection vaccine was needed. A modified diphtheria toxin was the obvious vaccine candidate and by 1915 Park and his associates had begun to use mixtures of toxin with antitoxin, in combination with Schick testing. The Schick test (1915) involved subcutaneous injection of a minute dose of toxin, and a negative result identified non-immune children. They were retested after vaccination to demonstrate test conversion. During the early 1920s Park and colleagues found four times more diphtheria in 90 000 matched unvaccinated children than in 90 000 vaccinated children [Reference Park, Schroder and Zingher6] a result that led to hundreds of thousands more Schick-negative children in New York and other American cities being given toxin-antitoxin mixtures up to 1927. The mixtures were, however, hard to standardize and potentially unstable. There was both underdosing and overdosing with the toxin/antitoxin mixture as well as incidents of vaccine contamination [Reference Paul7, Reference Wilson8]. In already sensitized recipients the serum component could cause fever, urticaria and joint pains, and even anaphylaxis.
Consequently, in the mid-1920s pioneer vaccinators' attention turned to modifying the diphtheria toxin by physico-chemical rather than serological means. The aim was ‘to immunise with maximum regularity after a minimum number of injections, which shall be followed by the least possible local and constitutional reactions’ [Reference Forbes4]. Fraenkel had shown that heating at 70°C for several hours removed toxicity, and Lowenstein had achieved the same effect by 4 weeks' exposure of the toxin to formalin. In 1924 the French investigator Gaston Ramon confirmed that prolonged incubation of filtrates of Corynebacterium diphtheriae cultures with formalin abolished their toxicity while preserving their immunogenicity [Reference Ramon9]. Ramon called his formol-toxoid vaccine ‘anatoxine’ and he gave it in two, later three, doses from 6 months of age [Reference Ramon10]. In Chicago, George and Gladys Dick compared formal-toxoid favourably with toxin/antitoxin [Reference Dick and Dick11]. Later a flocculent mixture of toxoid-antitoxin was found to be less reactogenic for older recipients than the toxoid alone.
Ramon was indefatigable as a protagonist of his anatoxine, and by the early 1930s it was well attested that the two toxoid vaccines, formol toxoid and toxoid/antitoxin floccules, safely met the needs of younger children and of older children who might be sensitive to animal serum or diphtheria protein. By then, too, comprehensive diphtheria prophylaxis was established in big American cities like New York (Table 1). Later the addition of alum, which in various formulations precipitated the toxoid and delayed absorption, was shown to make two doses of toxoid generally sufficient [Reference Fulton12].
Table 1. New York City: diphtheria mortality
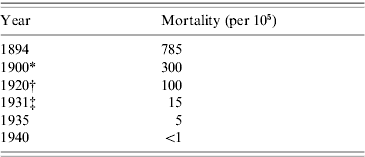
* Post introduction of antitoxin treatment.
† Post introduction of toxin/antitoxin immunization.
‡ Post introduction of toxoid immunization.
Unpropitious circumstances
In the UK, however, the anti-vaccination lobby was alert to any proposal to introduce mass immunization of children with multiple injections of a new vaccine, and such opposition was bound to give the public health authorities pause. Smallpox vaccination, which involved a virtually painless insertion of ‘lymph’, had from its very beginnings been opposed by some doctors and lay people; and from 1853, when legislation made infant vaccination mandatory, that resistance had grown ever stronger. By 1900 compulsory vaccination was so unpopular that its abolition was, for instance, an election manifesto commitment of the Independent Labour Party. The disappearance of epidemic smallpox from the UK after 1903 both encouraged non-compliance with and hardened opposition to vaccination [Reference Bayly13].
In the 1920s compulsory infant smallpox vaccination suffered a further setback when doctors learnt that it carried risks additional to the familiar ones of bacterial contamination and eczema vaccinatum. New data suggested that primary vaccination carried an incidence of disabling, sometimes fatal, encephalitis possibly as high as 1/10 000 [Reference Wilson8, Reference Dible14], and in the absence both of endemic and epidemic smallpox British Medical Officers of Health (MoHs) increasingly felt that they could rely on emergency ‘ring’ vaccination to control any outbreaks. It was not a propitious background against which to attempt the introduction of mass immunization of children against diphtheria.
John Fitzgerald, promoter of diphtheria toxoid
Outside the UK reservations about expanding the scope of immunization carried less weight. As far as the rest of the English-speaking world was concerned the most influential person in actuating public health officials to immunize against diphtheria with toxoid was probably the Canadian, John Fitzgerald. In Toronto in 1914 he had founded what became the Connaught Laboratories to prepare diphtheria antitoxin [Reference Rutty15] and, following a visit to Ramon in Paris in 1925, he instigated the manufacture of diphtheria toxoid at those laboratories [Reference Fitzgerald16, Reference Fitzgerald17]. They soon became a not-for-profit source of diphtheria toxoid for all of Canada.
Also in 1925, the University of Toronto had appointed John Fitzgerald head of a new School of Hygiene which attracted Rockefeller funding. The appointment enabled Fitzgerald to bridge the professional divide between those in commercial enterprises in various countries (UK included) who were modifying diphtheria toxin to produce vaccines and health officials whose job it was to persuade the public to accept these products. No one was given such a role in the UK – it was apparently beyond the power even of an eminent Chief Medical Officer (Sir George Newman) to promote a national programme of diphtheria immunization. Apart from anti-vaccination sentiment one explanation of this may have been the reports from abroad of residual diphtheria vaccine toxicity and of cross-contamination, the report closest to home being an episode of syringe-transmitted tuberculosis following some toxoid injections in Waterford, Eire, in 1937 [Reference Wilson8].
In Ontario, by contrast, Fitzgerald had been using diphtheria toxoid since 1926, and within 5 years had seen the incidence of diphtheria in Toronto fall by 90% [Reference Fitzgerald18]. Other Canadian provinces and various American cities followed suit. Ramon's toxoid was also being used in France, Belgium, The Netherlands and elsewhere [Reference Paul7], although it was probably the North American experience that was to be most influential in eventually persuading the British to adopt diphtheria toxoid immunization.
Refractory Britain
For the time being, though, there was a hiatus in UK, lasting into the 1940s. Consequently, with no routine diphtheria immunization in place, 2500–3000 children were dying of the disease each year. Ramon referred to ‘Angleterre refractaire’, and E. H. R. Harries, a leading English infectious diseases specialist, wrote despairingly in 1942 that the continuing morbidity and mortality from diphtheria ‘must seem fantastic to foreign observers’ [Reference Harries19].
So why throughout the 1930s and early 1940s did British practice remain at variance with that of the Canadians, the French, go-ahead United States health departments and the rest? The increasingly cautious attitude towards routine smallpox vaccination was only one of several factors contributing to a British reluctance to immunize children against diphtheria. Another reason may have been the reliability of notification of infectious disease and of death certification in the UK. This served to show that in spite of epidemic years there was an underlying decline in diphtheria incidence and mortality even in the absence of immunization. With arrangements already in place for removal and isolation of cases (isolation hospitals had been retained and costly new ones built since what turned out to be the final British smallpox epidemic of 1901–1903) the observed decline in diphtheria allowed, even perhaps encouraged, questioning of the need for mass immunization.
Ironically, the lively British academic interest in several aspects of diphtheria infection may also have delayed immunization. When, for instance, the toxoid vaccine first became available Park and his American followers had continued to Schick test before vaccinating. A debate ensued in the UK as to whether these tests for susceptibility were a necessary preliminary to selecting recipients of diphtheria toxoid (which they scarcely were). It was further suggested that incomplete immunization with toxoid might create carriers.
Then, in 1931, British bacteriologists described the characteristics of three C. diphtheriae types, gravis, intermedius and mitis [Reference McCleod20]. Isolates of the first and second types were most commonly the significant toxin producers, and much store was set by identifying their characteristic colonial differences. However, at the level of the individual isolate distinction from mitis strains was not predictive of toxigenicity and clinical severity, and until Elek introduced an in vitro procedure in 1949 it took a test of each isolate in guinea pigs to confirm that it was toxigenic [Reference Elek21]. What with Schick testing, tests for throat carriage and laborious tests for type and virulence, there were just too many reasons to investigate rather than get on and immunize with toxoid. In 1955 the authors of the fourth edition of the prestigious British textbook ‘Topley and Wilson’ were still observing somewhat patronisingly that: ‘in the United States some workers have failed to type their strains satisfactorily’. They would have done better to acknowledge that diphtheria had by then been banished from North American cities for so long that typing was no longer an issue.
Professional reluctance
Not only did British academic investigators lack the necessary focus and determination, but the leaders of the British medical profession also found reasons to eschew routine diphtheria immunization. A British Medical Journal review in 1932 observed testily that ‘the average MoH is unable to keep in touch with the voluminous and repetitive writings of M. Ramon’ [Reference Debre22]. Furthermore, the North American experience suggested that while children of school age might be easy to reach the elimination of diphtheria would depended on immunizing pre-school children as well [Reference Godfrey23]. Separate arrangements would be required for each group. Compared with the single superficial insertion of smallpox vaccine, moreover, the injections of diphtheria toxoid would be painful, and local reactions had sometimes been reported. Altogether, participation in mass diphtheria immunization was not an appealing prospect for the generality of British doctors whatever public health officials might recommend.
Administrative weakness
At the root of the apparent British indifference to the continuing incidence of childhood diphtheria, though, were administrative arrangements that delegated decision-making to individual MoHs. They had to rely on their local authorities to fund mass immunization, and these municipalities, already burdened with dispensaries and infant welfare clinics, were mostly disinclined to incur another expense. MoHs therefore had responsibility without power. In 1931 Forbes estimated that only 1·12% of Scottish and 0·35% of English and Welsh children were immunized against diphtheria [Reference Forbes4]. Later in the 1930s a few larger municipalities did fund some use of diphtheria toxoid; but otherwise only special circumstances saw groups being immunized. British fever hospitals, whose nurses often contracted diphtheria, had begun to immunize their staff as early as 1923 [24]; and between 1928 and 1931, as part of a Medical Research Council-funded study, 1000 boys living in close proximity in the residential Greenwich Hospital School were given diphtheria toxoid (it virtually eliminated the disease from the school for those 4 years) [Reference Sheldon25]. Privately funded vaccinations also took place; but immunization of whole communities was neglected in spite of growing international evidence that once three-quarters of children had been vaccinated a ‘herd’ immunity was established. So, while vigorous immunization campaigns were being pursued elsewhere, the UK dithered. Anti-vaccination feeling and awareness of accidents abroad following diphtheria immunization were continuing negative influences: neither politically nor professionally was there the appetite to intervene.
The impetus of war
The outbreak of war in Europe in September 1939 was followed by months of ‘phoney’ war during which frenetic preparations were made for the expected consequences of total war such as aerial bombardment of civilian populations. Government assumed responsibility for the safety, nutrition and health of children to an extent that would previously have been unthinkable. When, early in 1940, a Lancet editorial asked why the existing measures of notification, removal and disinfection (steps which had manifestly failed to control diphtheria) were not being supplemented by a programme of diphtheria immunization in the UK the editor must have known that he was pushing at an open door [26]. Ministry of Health circular 1307 (1940) had just recommended that the child population be immunized against diphtheria and for the first time Government was offering local authorities financial help towards the cost of immunization. This eventually led to diphtheria becoming a less common disease in the UK than in the disrupted parts of post-war continental Europe where diphtheria immunization had already been underway before 1940.
At first logistical difficulties arose, e.g. in obtaining consent to immunize evacuated children; and some parents were reluctant to have the vaccine given (‘but didn't they know there was a war on?’). Nevertheless, the speed of implementation was startling and in five towns almost half of the children aged 0–15 years had already been fully immunized by the end of 1941 [Reference Hartley27]. When in 1942 the Committee for the Study of Social Medicine investigated parents' reasons for not having their children immunized lack of facilities was rarely pleaded [Reference Paul7]. Although some towns dragged their feet a third of all children under 15 in England and Wales, and a half in Scotland, had been protected from diphtheria by the end of 1942.
By assuming unprecedented powers to protect children in 1940, and by daring to add another immunization to the often unpopular smallpox vaccination Government fostered a spirit of social solidarity in matters of Child Health which still survives. Any opposition to the diphtheria immunization was muted, perhaps because it would have seemed unpatriotic to leave at risk children huddled in air raid shelters or displaced by evacuation. The arguments in favour of diphtheria immunization were in any case strong, and much stronger than those for routine smallpox vaccination. The only serious medical complication of toxoid immunization to emerge was the rare occurrence of post-injection poliomyelitis [28]. This caused diphtheria immunization to be postponed in polio epidemic summer months in the late 1940s, but it had no lasting impact on uptake.
By the close of the Second World War the evidence of success was clear. What had been achieved in North America and elsewhere a decade and more earlier was being replicated in the UK. Childhood death rates from diphtheria fell from 38·5/100 000 in 1934 to 9·2/100 000 in 1944; by 1949 mortality and morbidity had both fallen more than tenfold from pre-war levels (Fig. 1).
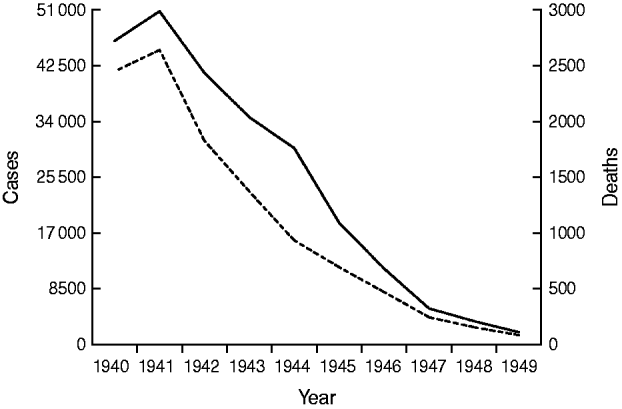
Fig. 1. Diphtheria cases (——) and deaths (- - -), England and Wales, 1940–1949.
Discussion: the further expansion of childhood immunization
Diphtheria immunization in the UK, while much delayed, set the pattern for subsequent introductions of childhood immunizations, a policy strengthened by the terms of the 1948 National Health Act. Squeamishness about multiply injecting children was overcome to a remarkable degree and parental consent was very generally given. Acceptance rates, which had often been far from complete in those countries where diphtheria immunization was regularly practised in the 1930s, reached 90% in parts of post-war Britain. Immunization of the appropriate age groups was introduced against tetanus and whooping cough, tuberculosis and poliomyelitis, and measles, mumps and rubella. More recently Haemophilus influenzae B and then meningococcus C and papilloma virus vaccines have been added [Reference Salisbury, Ramsay and Noakes29]. Additional childhood immunizations, e.g. against chickenpox, rotavirus and hepatitis A and B are conceivable depending on the outcome of cost-benefit analyses and the availability of funds.
Although there has been continuing debate about schedules, safety, acceptability, adequacy of uptake and value for money, though each potential addition has been viewed with circumspection in some quarters, and though there have been a few false starts, the British childhood immunization programme has continued to expand. More than once a lone academic voice has challenged an element of the programme already in place [Reference Baker30–Reference Bedford and Ellman32] and this has grossly perturbed compliance rates; but after careful evaluation these criticisms have been refuted. Regrettably, though, their negative effect on compliance, and the predictable consequences in terms of avoidable morbidity, have outlived the criticisms.
Funding for the immunization programme has continued to be central not local, and ministerial decision making is still guided by a single expert committee [Reference Hall33]; both of these have been factors preserving consistency in policy making. Health budgets have recently been devolved within the UK, but the programme has so far remained uniform. Diphtheria immunization itself has been an unqualified success. Children have been fully protected at their most vulnerable age, toxigenic strains of C. diphtheriae have virtually disappeared from circulation and carriers of them are rarely found. The only remaining threat is from occasional importations. However, experience has shown that it is harder and sometimes impossible to attain the same degree of success against every pathogen. In particular, repeated immunizations may be needed, something which, incidentally, Jenner resisted throughout his lifetime with regard to his own great discovery of smallpox vaccine.
The general lessons learnt since Britain's delayed engagement with diphtheria immunization have been these: study as far as possible the natural history of the disease in question and consider how the expected uptake of a new vaccine may modify it; consider what a candidate vaccine can be expected to achieve over recipients' lifetimes; carefully weigh up costs, financial and other, against proven and likely benefits. The diphtheria toxoid vaccines, when finally introduced in UK, ticked these boxes; but whether the same can be said of every candidate vaccine possibly now coming under consideration, and whether further expansion of the programme of childhood immunization is sustainable remain important questions which are beyond the scope of this paper. While an historical viewpoint like the present paper's cannot point the path to the future those who deal with the detail of immunization policy do need to have a long-term perspective. Any future development of the programme should be based on the fullest possible epidemiological understanding of each infection targeted, an estimate of how likely it is that a new vaccine will confer a lifetime's immunity on recipients and a consideration of what the impact will be on the overall prevalence of the disease if the vaccination is sustained.
Conclusion
In 1940, British Ministry of Health circular 1307 reversed policy, sweeping away the professional and political inertia that had delayed mass immunization against diphtheria. This was, in the words of the title above, a ‘debacle’ (in its original sense of a sudden thaw in a stream pent up by ice). Circular 1307 also set the pattern for the present comprehensive programme of childhood immunization in the UK. The wisdom of determining and funding that programme centrally has since become plain, but deciding just how far it can expand requires a long-term perspective on expected benefits and weighing these up against cost and sustainability. The programme was first conceived at a time of national crisis and then nurtured by a sense of social solidarity. Its future depends on coherent and consistent policy making, a strong evidence base that will maintain parental confidence, and adequate funding to sustain immunizations that already have and may yet be initiated.
DECLARATION OF INTEREST
None.






