INTRODUCTION
Since the inception of the Global Polio Eradication Initiative in 1988 under the auspices of the World Health Assembly [1], significant advances towards achieving this objective have been met. The most notable results have been the declaration of the eradication of polioviruses from the Americas (1994) [2], the Western Pacific region (2000) [3] and Europe (2002) [4]. Four countries are still continuing to present endemic circulation of wild-type polioviruses and others are regularly re-infected [5]. Doubts are emerging regarding when, and even if, this eradication is achievable with the current strategy [Reference Chumakov6–Reference Vashishtha11]. Even in regions now free of wild-type poliovirus circulation, cases of poliomyelitis disease have continued to occur due to vaccine-derived polioviruses (VDPVs) originating from oral poliovirus vaccine (OPV) vaccinees [Reference Agol12, Reference Kew13]. These VDPVs have been at the origin of several localized outbreaks [14, Reference Kew15].
When cases of vaccine-associated paralytic poliomyelitis (VAPP) become more frequent than those due to wild-type virus, it is time to consider ‘endgame’ strategies to achieve true eradication of poliovirus-induced diseases. Several workgroups established by the WHO or by national authorities in countries where wild-type virus circulation has ended have recommended alternative vaccination strategies, ranging from the complete cessation of OPV administration to the complete replacement of the use of OPV by the inactivated poliovirus vaccines (IPV), or by the adoption of mixed or sequential IPV/OPV schedules [16–Reference Grassly20]. These scenario have been debated intensely, and several arguments are under consideration: those from groups stating that IPV should be routinely and indefinitely implemented at worldwide level [Reference Chumakov6], those from groups basing their arguments on the cost/effectiveness of IPV-based strategies that will make the different scenarios more or less feasible according to the different assumptions made regarding IPV vaccine costs [Reference Griffiths, Botham and Schoub21–Reference Thompson24], and those basing their arguments on the localization and origin of industrial capacity and its development making the scenario more or less attractive from geopolitical perspectives.
Following the declaration that South America was polio-free in 1994 [2], some local health authorities in the region began to consider the cessation of OPV vaccination and the introduction of routine IPV vaccination, either in a sequential schedule (mixed use of IPV and OPV) or an IPV-only schedule. One such region is Cordoba Province, Argentina, where the local health authorities plan to replace OPV by IPV in the routine infant immunization schedule. The proposal was to introduce IPV (through the use of a DTwP-IPV-Hib pentavalent combination vaccine) in a sequential three-dose primary series schedule at ages 2, 4, and 6 months followed by a booster dose of OPV at 18 months. However, during the course of our study it was decided to implement an IPV-only schedule with complete replacement of OPV by IPV.
In accordance with WHO guidelines [16] and with the objective of evaluating the programmatic feasibility and value of such a vaccination programme, an epidemiological survey was established in Cordoba City before the initiation of this vaccination programme to determine the overall seroprevalence of poliovirus antibodies in infants aged 8–12 months following their routine primary vaccination series, and in children aged 19–21 months following their routine booster vaccination. This initial survey was followed by a second survey in the same age groups 1 and 2 years after the initiation of this new vaccination programme in Cordoba City, and the results of this second survey were compared to the results of a parallel survey done in the same age groups and at the same time intervals in two control areas that continued to use an OPV-only schedule.
MATERIAL AND METHODS
This was a prospective descriptive study with several cross-sectional seroprevalence surveys in three cities of the Cordoba Province, Argentina (April 2002 to January 2005). The main objective was to assess vaccination coverage and immunity to polioviruses in two age groups (8–12 months and 19–21 months) in areas where a primary series and booster vaccination schedule with IPV has been introduced in place of an OPV-based polio vaccination schedule, compared to contiguous areas continuing to use OPV.
The study area was the city of Cordoba and its surrounding area, where an IPV combination vaccine was introduced at ages 2, 4, and 6 months with a booster dose at 18 months. The neighbouring cities of Villa Maria and Rio Cuarto in the inner Cordoba Province continued to use a full OPV schedule and served as control areas.
Participants were infants and children in the requisite age groups (8–12 and 19–21 months) living in the study areas since birth. Children with any known immunological deficiency (including HIV infection) or having received immunosuppressive therapies within the previous month, those with an acute illness at the time of the visit or any history of blood or blood component transfusion within 3 months were excluded. Informed consent was obtained from the parents or guardians of all participants. Approval was obtained from the Cordoba Province's Ministry of Health, and the study was conducted according to the Declaration of Helsinki principles as amended in October 1996, Republic of South Africa [25].
Study schedule
Two groups of infants aged 8–12 months (after first vaccination) were randomly selected in the study intervention area first prior to (April–October 2002) and then 1 year after (February–March 2004) the initiation date of the DTwP-IPV-Hib vaccination programme (January 2003) (group A1: three-dose primary OPV series schedule at ages 2, 4, 6 months; group A3: three-dose primary IPV series schedule at ages 2, 4, 6 months), compared to two groups within the control areas where OPV usage was maintained throughout the full study period (group B1: February–November 2002; group B3: January–June 2004).
Seroprevalence was also monitored in randomly selected children aged 19–21 months in the study intervention area before (January–December 2002) and then 2 years after (December 2004 to January 2005) the initiation date of the DTwP-IPV-Hib vaccination programme (group A2: three-dose primary OPV series schedule at ages 2, 4, 6 months and a booster dose of OPV at 18 months; group A4: three-dose primary combination IPV series schedule at ages 2, 4, 6 months and a booster dose of IPV alone at 18 months), compared to two groups within the control areas where OPV usage was maintained throughout the full study period (group B2: May–November 2002; group B4: December 2004 to January 2005).
Intervention study vaccines
In Cordoba City, the pentavalent DTwP-IPV-Hib vaccine (Pentact-Hib®, Sanofi Pasteur, France) containing a minimum of 40 IU tetanus toxoid (TTd), 30 IU diphtheria toxoid (DTd), 4 IU B. pertussis organisms, 40 D-antigen units (U) poliovirus type 1, 8 U poliovirus type 2, and 32 U poliovirus type 3, and 10 μg polyribosylribitol phosphate Haemophilus influenzae type b conjugated to 10–20 μg TTd was used. Each vaccine dose was injected intramuscularly. From April 2004 onwards, due to a lack of supply, the polio vaccination intervention programme switched to the use of a stand-alone IPV vaccine (Imovax Polio®, Sanofi Pasteur) which contained a minimum of 40 D-antigen units (U) poliovirus type 1, 8 U poliovirus type 2, and 32 U poliovirus type 3.
Before the vaccine intervention study and within the control area, OPV was used.
The poliovirus vaccines were used according to the national vaccination recommendations, i.e. three doses given at ages 2, 4, and 6 months followed by a booster dose given around 18 months. Additionally, the booster at age 5–6 years was also with IPV in Cordoba City and surroundings.
Survey procedure
Vaccine coverage and seroprevalence data before and after introduction of IPV were collected for children who were selected by going from house to house in randomly selected administrative divisions of the intervention and control areas.
A standardized questionnaire was used in interviews with parents/guardians to determine each child's medical history to ensure adhesion to the inclusion/exclusion criteria. Vaccination histories were determined from children's vaccination cards. If the card was not available, the parents were asked additional questions, particularly with reference to ‘vaccine given by mouth’, and the number of such vaccine doses. A child was considered as vaccinated after having received a full three-dose primary series schedule (groups aged 8–12 months) completed by a fourth dose of booster (groups aged 19–21 months), irrespective of the differences between recommendations and real age at vaccination.
Poliovirus antibody determination
A 3-ml blood sample was taken from each participant, and sera were prepared and stored at −20°C within 8 h. All sera were analysed in a blinded manner at the end of the study at the Institute of Virology, Cordoba University, Argentina. Neutralizing poliovirus antibodies were assessed by micro-neutralization assay following the modified WHO method with wild poliovirus strains used as challenge virus [26]. Sera were inactivated by heating at 56°C for 30 min, and twofold serial dilutions (from 1:4 to 1:8192) tested against the three reference poliovirus serotypes (Mahoney, MEF-1, Saukett). Duplicate dilutions (50 μl) of serum samples were mixed with equal amount of virus suspension containing 100 TCID50 for each poliovirus and incubated for 3 h at 37°C in a 96-well microcultured plate at 5% CO2 atmosphere. Then 100 μl Hep-2 Cincinnati cell suspension (160 000 cells/ml) were added to each cell and incubated for 6 days. The anti-poliovirus 1, 2 and 3 serum neutralizing antibody titre was the reciprocal of the highest dilution that protected 50% of the cultured cells against 100 TCID50 of the challenge virus on duplicate wells. A titre of ⩾1:8 was considered protective.
Statistical methods
Assuming a seroprotection rate of 85% for each polio serotype, an estimated 200 participants were required for each age group to estimate the seroprevalence rate with a 95% level of confidence (±5%). The planned sample size was therefore 250 children per group.
Results are reported descriptively for vaccine coverage and seroprevalence rates. All individual titres >8192 (1/dilution) were set to 8192 for geometric mean titre (GMT) calculations. For each individual survey and each age segment, the proportion of seropositive (seroprotected) participants and their GMT of antibody against the three poliovirus strains were calculated [with their respective 95% confidence intervals (CI)]. The seroprevalence rates for poliovirus antibodies were compared in the two age groups between the intervention area and the control area using χ2 test (or Fisher's test if the calculated sample size was <5) with P values <0·05 considered significant. Data were analysed with SAS software version 9.1 (SAS Institute, USA).
RESULTS
Study areas and demographics
The intervention area of Cordoba City has a population of 1 600 000 inhabitants, with an annual birth cohort of about 30 000 (2500 per month). At any given time, there are around 10 000 children aged between 8 and 12 months and 5000 aged between 19 and 21 months. The only cities in the same region with around 200 births per month are Villa Maria (population 76 000) and Rio Cuarto (population 160 000) with about 160 and 220 births per month, respectively. Thus, at any one time there are around 1520 children aged 8–12 months and 760 aged 19–21 months in these two cities.
In our study, 836 participants aged 8–12 months (398 pre-IPV, 438 post-IPV introduction) and 771 aged 19–21 months (348 pre-IPV, 423 post-IPV introduction) were randomly selected from the respective age groups in the participating cities. Demographic data are shown in Table 1. Overall the participants were older and the age range wider than intended in all groups except for the 8–12 months pre-intervention age group in Cordoba City. This group tended to be younger than the pre-intervention control area group [age (mean±s.d.) of group A1: 10·4±2·2 months; group B1: 13·4±3·8 months]. However, 1 year later, after the introduction of IPV, the mean ages of the post-intervention group participants were similar in the two areas. Mean ages were similar in children (19–21 months target age) from both areas prior to and after the intervention.
Table 1. Demographic data of participants
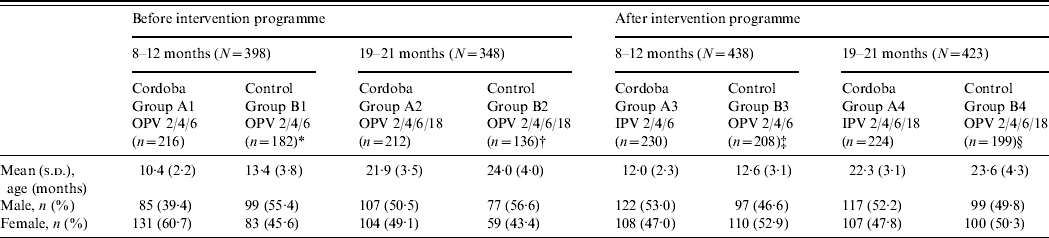
A1, B1, B3: Infants who had received three-dose primary OPV series schedule at ages 2, 4, and 6 months (OPV 2/4/6).
A2, B2, B4: Infants who had received three-dose primary OPV series schedule at ages 2, 4, and 6 months and a booster dose of OPV at 18 months (OPV 2/4/6/18).
A3: Infants who had received three-dose primary IPV series schedule at ages 2, 4, and 6 months (IPV 2/4/6).
A4: Infants who had received three-dose primary IPV series schedule at ages 2, 4, and 6 months and a booster dose of IPV at 18 months (IPV 2/4/6/18).
* Rio Cuarto (n=114), Villa Maria (n=68).
† Rio Cuarto (n=102), Villa Maria (n=34).
‡ Rio Cuarto (n=105), Villa Maria (n=103).
§ Rio Cuarto (n=105), Villa Maria (n=94).
Vaccination coverage
Information from vaccination cards was more readily available in Cordoba City than in the control areas. Vaccination cards were provided for the majority of participants in Cordoba City except for the 19–21 months pre-intervention children (group A2). Pre-intervention vaccination history of participants in the control areas was predominantly collected from parental interviews. Post-intervention vaccination cards were available for the majority of participants in all areas (Table 2). When further information was obtained by parental interviews, the reported vaccination histories were similar to the recorded ones in all areas (results not shown). In Cordoba City, prior to the introduction of IPV, 80·1% of children aged 8–12 months and 60·4% aged 19–21 months had been fully vaccinated according to vaccination cards and parental interview; the corresponding rates were 95·6% and 87·5%, respectively, in the control areas. Overall, there was higher vaccine coverage in the control areas than in Cordoba City before implementation of the IPV programme [only two children (one in each area) had never been vaccinated]. One year after the introduction of IPV vaccine in the study area, the coverage rate in Cordoba City had increased to 92·6% in children aged 8–12 months and 93·3% in those aged 19–21 months (with only two children never vaccinated), while the rate in the control area still administering OPV fell to 87·5% in the 8–12 months group and was unchanged (83·9%) in the 19–21 months group (with 23 children never vaccinated).
Table 2. Number of participants vaccinated (n) from total participants (N) included in the survey according to information source
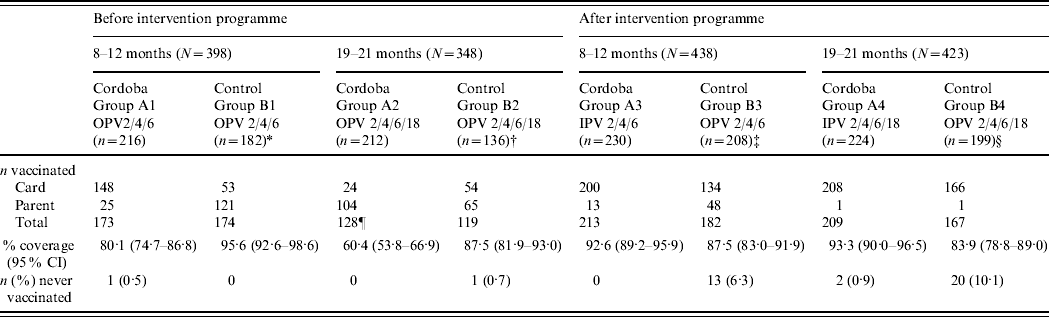
* Rio Cuarto (n=114), Villa Maria (n=68).
† Rio Cuarto (n=102), Villa Maria (n=34).
‡ Rio Cuarto (n=105), Villa Maria (n=103).
§ Rio Cuarto (n=105), Villa Maria (n=94).
¶ Two participants had missing information source data.
Poliovirus antibody seroprevalence
Baseline seroprevalence of antibodies against the three poliovirus types was determined in both intervention and control areas immediately before implementation of the DTwP-IPV-Hib vaccination programme in Cordoba City then 1 year and 2 years after its implementation. Seroprevalence rates for antibodies against the three poliovirus types observed in the eight study groups are shown in Figure 1. Seroprotection rates against poliovirus types 1 and 2 were consistently above 97% in both Cordoba City and in the control areas of Rio Cuarto and Villa Maria, before and after the intervention programme and in the 8–12 and 19–21 months groups. Seroprotection rates against poliovirus type 3 were lower, ranging between 89·3% and 97·0% before the intervention programme and increased significantly at 1 year (8–12 months group: 89·3–96·9%, P=0·002) and 2 years (19–21 months group: 92·3–99·6%, P<0·001) after the introduction of DTwP-IPV-Hib in Cordoba City. In the control area, the seroprevalence rates were higher pre- than post-intervention in the 8–12 and 19–21 months groups, but the differences were not significant.

Fig. 1. Seroprevalence rates for antibodies against the three poliovirus types in the respective groups and areas, before and after implementation of the DTwP-IPV-Hib programme in Cordoba City. □, Cordoba City; ![]() , control areas.
, control areas.
Seroprevalence rates in Cordoba City post-intervention were equivalent or better than the seroprevalence rates of the control area post-intervention. The differences were significant for type 3 in both the 8–12 and 19–21 months groups (P=0·006 and P=0·003, respectively). The difference was also significant for type 1 in the 19–21 months age group (P=0·03).
Overall seroprevalence rates defined as being seropositive for all three types of poliovirus are shown in Figure 2. The overall seroprevalence increased slightly in the study area and decreased slightly in the control areas post-intervention. The highest rate (99·5%) was observed in the 19–21 months post-intervention group in Cordoba City and the lowest (85·4%) was recorded in the 8–12 months post-intervention group in control areas.
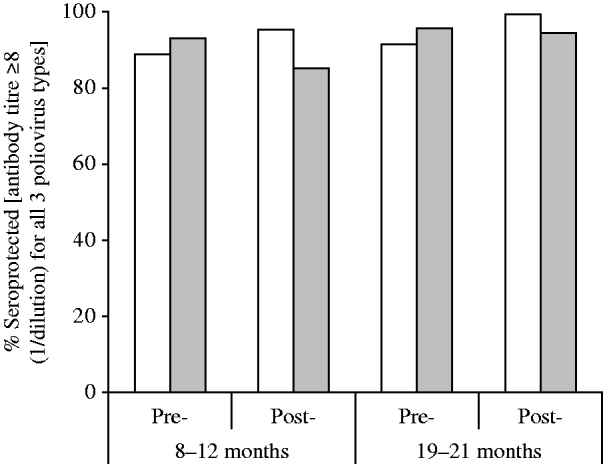
Fig. 2. Overall seroprevalence rates (participants seropositive for all three poliovirus types) by group, before and after implementation of the DTwP-IPV-Hib programme in Cordoba City. □, Cordoba City; ![]() , control areas.
, control areas.
Antibody titres (Fig. 3) reflect the differences in seroprevalence rates. GMTs against all three poliovirus types were essentially the same in the study and control areas before the implementation of the intervention programme. The slightly higher levels in the control area probably reflect the older mean age of these participants. The introduction of DTwP-IPV-Hib vaccine in Cordoba City resulted in around a twofold increase in antibody titres against type 1 and over sevenfold increase for type 3 in infants aged 8–12 months. Conversely, titres against type 2 post-intervention in Cordoba City were about 60% of those pre-intervention in this age group.
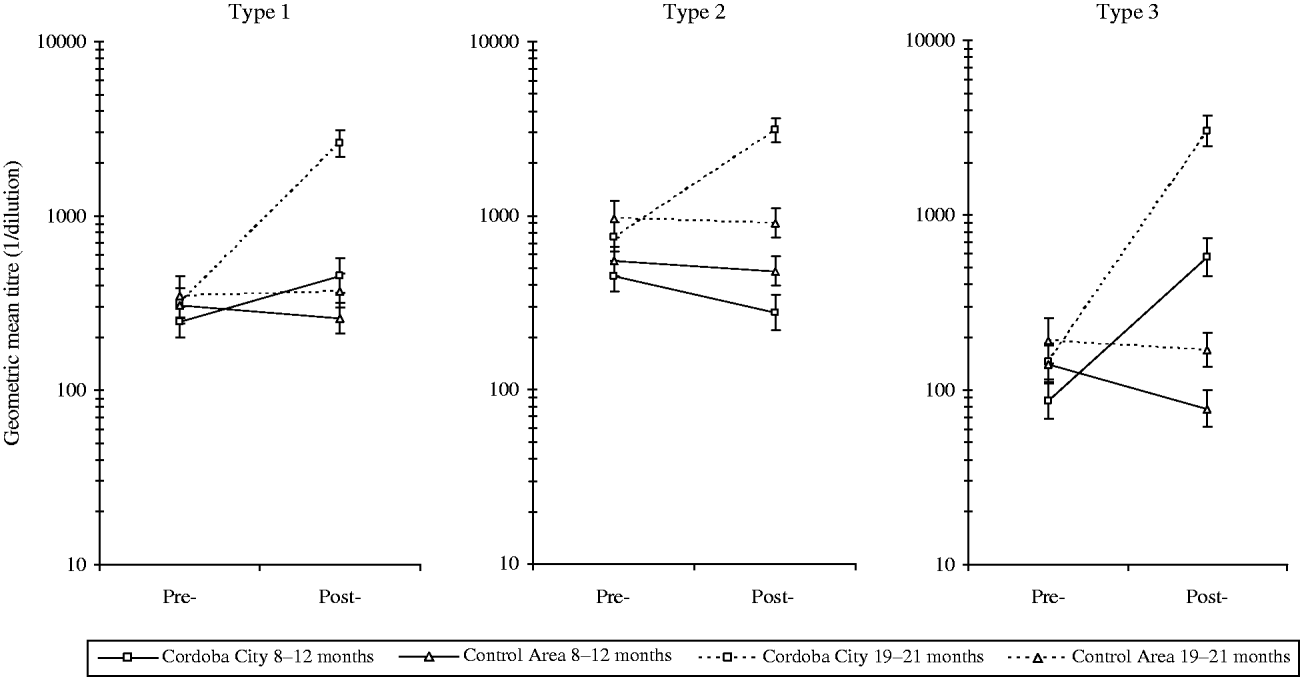
Fig. 3. Geometric mean titres of antibodies to poliovirus types 1, 2, and 3 before and after implementation of the DTwP-IPV-Hib programme in Cordoba City.
In children aged 19–21 months in Cordoba City, increases of about 8-, 4-, and 21-fold against poliovirus types 1, 2, and 3, respectively, were observed. In the 19–21 months controls, GMTs remained at similar levels in both groups with a slight reduction of GMTs except for type 1 poliovirus.
DISCUSSION
As more regions become officially ‘polio-free’ there is increasing pressure to end the use of OPV vaccines, which themselves are becoming the primary cause of poliomyelitis in such regions. Proposed strategies to halt OPV use while maintaining seroprotection in the population against the remnants of the circulating virus include the use of IPV vaccines in full or sequential schedules. Following this strategy, the health authorities of Cordoba Province were looking to replace OPV by an IPV-only schedule administered as an IPV-containing combination vaccine. The present survey was performed in order to determine the current status of OPV vaccine usage and seroprotection and to assess the effect of the proposed replacement of OPV with an IPV-containing combination vaccine in the primary immunization schedule. Regions such as Latin America, where wild-type poliovirus is no longer in circulation, are now faced with the dilemma that the only cases of polio infection are due to viruses derived from OPV. While cessation of polio immunization may be an attractive option, it is not a viable one because of the possibility of the continued shedding and circulation of VDPV. There is evidence that such shedding can continue for several years from persons with immune deficiencies [Reference Minor27] and that this could trigger poliomyelitis disease due to VDPVs in the new immunologically naive populations. Outbreaks in Egypt, Hispaniola, the Philippines and Madagascar have been shown to have originated from vaccine-derived viruses [14, Reference Kew28].
The WHO has proposed two ‘endgame’ strategies involving IPV to ensure continued protection while eliminating OPV and consequently the risk of VAPP. The authorities in Cordoba City had originally intended to implement one of the strategies, the use of IPV and OPV in a mixed schedule, but during the course of our study decided to use an IPV-only schedule.
These first results from the seroprevalence surveys performed before and 1 year after the introduction of a full three-dose primary schedule of DTwP-IPV-Hib demonstrate one unexpected advantage – an increase in vaccine coverage – while maintaining the immune protection against poliovirus types 1 and 2, and increasing it against poliovirus type 3. Prior to the implementation of the IPV immunization programme, vaccine coverage in the study area of Cordoba City (~70% in both groups combined) was lower than in the two smaller cities of Rio Cuarto and Villa Maria used as controls (~92%). After 1 year of DTwP-IPV-Hib immunization, polio vaccine coverage in Cordoba City increased to 93%, more than the rate in the control areas still using OPV which had fallen to ~86% in both groups combined. This increase was probably a real effect as vaccine coverage rates were similar when collected from written (vaccination cards) or verbal (parental interviews) accounts. Increased coverage was not associated with increased seroprevalence of antibodies against poliovirus types 1 and 2, which were already very high. This might be explained partly by our definition of vaccine coverage that corresponded to the administration of a complete vaccination series schedule. The majority of the children who did not complete their series received at least one dose of polio vaccine and might also have been protected. The seropositivity rate against type 3 increased from 89·3% to 96·9% in the 8–12 months group, concomitantly the sevenfold higher GMT was comparable with that for participants in the control area.
One year after the introduction of a three-dose primary schedule with a pentavalent IPV-containing combination vaccine in Cordoba City, seroprevalence rate and GMT against poliovirus types 1 and 2 was maintained and increased against poliovirus type 3 in infants aged 8–12 months. Seroprevalence and GMT against all three types increased in the 19–21 months group. Vaccine coverage in the study area also increased. The replacement of OPV by IPV in routine infant immunization as part of an effective endgame strategy could help eradicate the circulation of polioviruses in South America and ultimately worldwide.
ACKNOWLEDGEMENTS
The study was funded by Sanofi Pasteur. Keith Veitch, Simon Jones, and Andrew Lane (Sanofi Pasteur) provided support for preparation of the manuscript. The authors are grateful to Denis Crevat from the Sanofi Pasteur Global Clinical Immunology platform (at the time of the study) for the serological analyses, and to Emmanuel Vidor (Sanofi Pasteur) for helpful discussions during manuscript development.
DECLARATION OF INTEREST
Keith Veitch was an employee of Sanofi Pasteur at the time of his involvement in the manuscript preparation. Simon Jones and Andrew Lane are employees of Sanofi Pasteur.









