Cryptosporidiosis is an important cause of gastroenteritis worldwide, and New Zealand has one of the highest reported rates in the world with between 26·1 and 32·3 new cases per 100 000 population per year [Reference Learmonth1]. However, not all cases of cryptosporidiosis in the community are reported to national surveillance and so these numbers are likely to be significant underestimates [Reference Adak, Long and O'Brien2]. Cryptosporidiosis is caused by infection with protozoan parasites of the genus Cryptosporidium and cases have symptoms of gastroenteritis which typically last from several days to several weeks. Our understanding of the causes of cryptosporidiosis have highlighted risks from poorly treated drinking water, swimming in swimming pools, contact with farm animals and person-to-person transmission as being important [Reference Hunter3].
In many countries cryptosporidiosis incidence varies from year to year, and climatic variability and community spread from imported travel cases are suggested as important sources of this inter-annual variability [Reference Curriero4, Reference Lake5]. Precipitation may wash Cryptosporidium from the land into rivers or lead to effluents bypassing sewage-treatment works and being discharged directly into surface waters. If these waters are subsequently used for public water supplies then human cryptosporidiosis may result. Weather may also affect countryside trips and other recreational activities (e.g. swimming pool visits) potentially bringing humans into contact with Cryptosporidium. Imported travel cases may lead to secondary transmission.
This research aimed to improve the understanding of cryptosporidiosis in New Zealand through a detailed analysis of its seasonal patterns, and the factors underlying these. The analysis was undertaken at a national level, an approach which has been used in a large number of previous time-series studies [Reference Lake5–Reference Kovats7]. This is the first such study in New Zealand.
The number of monthly reported cryptosporidiosis cases in New Zealand between 1997 and 2005 were obtained, and any cases reporting recent foreign travel (4·3% of the total cases) were excluded as the infection may have been acquired abroad. It is known that these reported figures represent a small fraction of the total cases in the community but because ascertainment is unlikely to vary by month, the data should provide a representative time-series profile of cryptosporidiosis in New Zealand. Outbreak cases were retained in the analysis. These numbers were divided by an estimate of the New Zealand population each year (obtained from Statistics New Zealand) and the number of days in each month to produce a daily rate for each month. In order to investigate the seasonality of cryptosporidiosis in New Zealand, monthly graphs of incidence were constructed for the whole of New Zealand. Separate graphs were constructed for the North and South Islands (see Fig.).
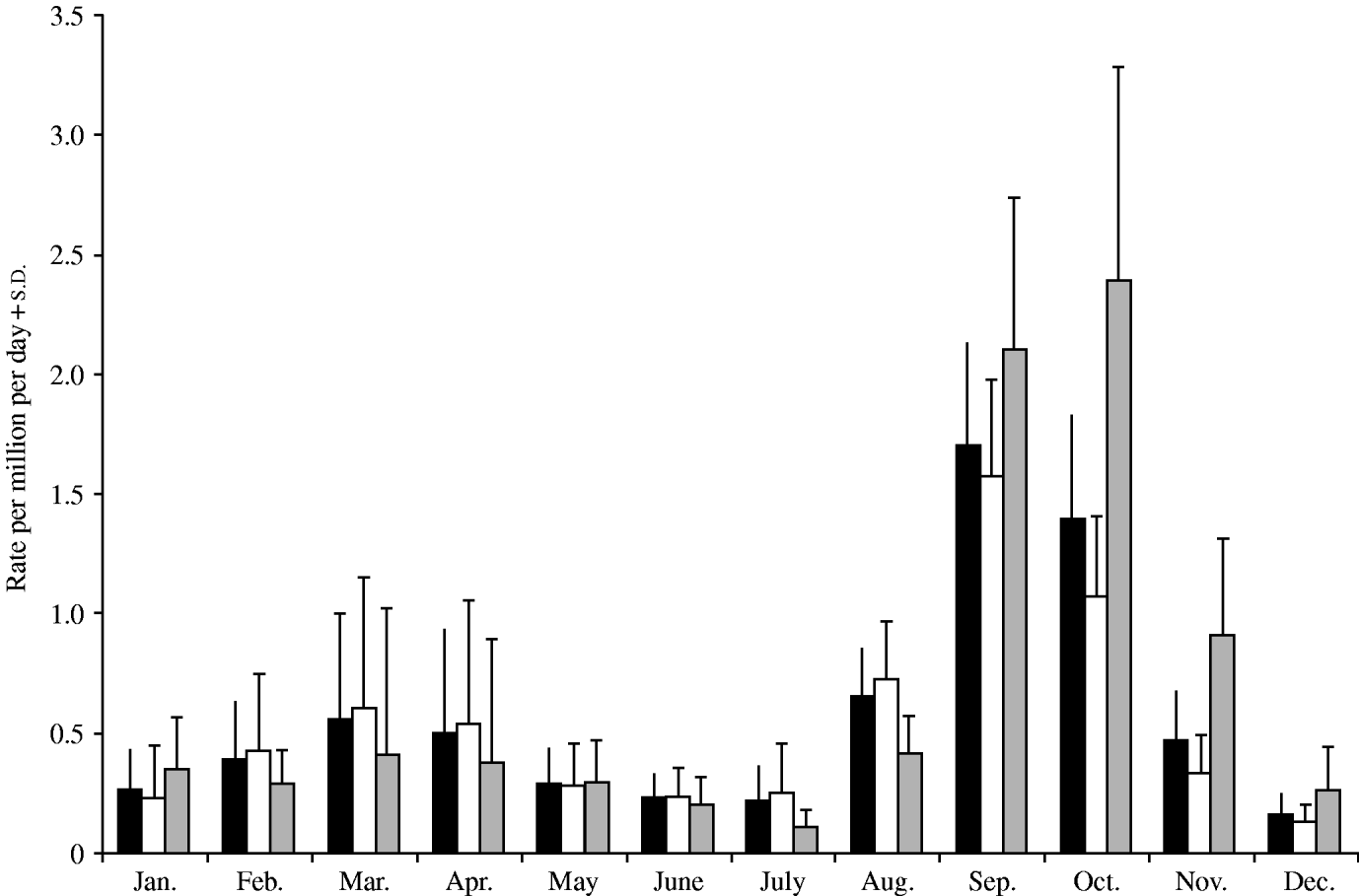
Fig. New Zealand average monthly cryptosporidiosis rate 1997–2005. ■, All New Zealand; ![]() , North Island;
, North Island; ![]() , South Island.
, South Island.
To account for possible causes of inter-annual variability in cryptosporidiosis incidence, mean monthly temperature was obtained for each month between 1997 and 2005 from the New Zealand National Climate Centre. This average temperature value is based upon temperature observations across New Zealand. Estimates of run-off were obtained using daily discharges for seven major rivers in New Zealand (Mohaka, Hutt, Mangakahia, Clarence, Waimakariri, Pomahaka and Buller). These were selected because of the continuous nature of the monitoring data, their geographical spread, and their mostly uncontrolled catchments. These data were standardized by average river flow, and averaged to produce one estimate of monthly river flow across New Zealand. Monthly rainfall was obtained for the 10 main urban centres in New Zealand and averaged to produce a single national estimate of monthly rainfall. The number of reported travel-related cryptosporidiosis cases each month was also obtained.
The possible associations between cryptosporidiosis and these explanatory variables were examined using previously published time-series regression methods [Reference Lake5, Reference Lake8]. These associations may vary at different times of the year. Consequently, the data were analysed by taking each month in turn and using ordinary least-squares regression to examine the relationship between the monthly cryptosporidiosis rate and the temperature, precipitation, river flow and travel cases in that month. To account for possible lagged relationships, variables were constructed indicating the values for the three previous months, which were then tested in the model. A 3-month period was selected because it is unlikely that a case of cryptosporidiosis will be affected by events before this period. It is not possible to assume that the monthly cryptosporidiosis rates are independent in time and therefore an autoregressive term was included in the model; specifically the cryptosporidiosis rate in the previous month. Results for individual months were then combined based on those with similar seasonal patterns and statistical results. This approach increased the degrees of freedom and consequently the statistical robustness of the results.
This procedure derived two time periods, January to May and June to December. When the data from individual months were combined it became important to control for seasonality, and therefore all models incorporated dummy variables for each of the individual months. The results for the models of inter-annual variability indicated that between January and May cryptosporidiosis was significantly (P<0·001) associated with mean New Zealand temperature in the current and previous month (Table). Similar results were obtained when data for the North and South Islands of New Zealand were analysed separately. No associations between cryptosporidiosis and the explanatory variables existed at other times of the year.
Table. Ordinary least-squares regression model of monthly cryptosporidiosis rate (cases/million population per day) between January and May
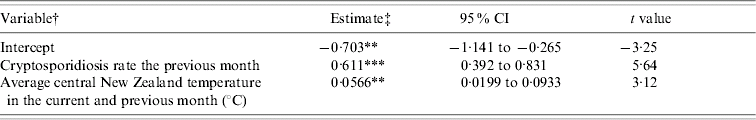
† The model also included 5 presence/absence variables indicating to which month each observation was a member.
‡ The change in cases in monthly cryptosporidiosis rate per unit change in the explanatory variable.
** P<0·01, *** P<0·001.
R 2=64·93%.
Degrees of freedom=39.
The discussion of these results will start by focusing upon the seasonal patterns of cryptosporidiosis revealed in the Figure. New Zealand displays two peaks of cryptosporidiosis incidence, one in the spring (September) another in the autumn (March). This seasonal pattern mirrors that of England and Wales [Reference Lake5] whereas other countries, such as the United States [Reference Hlavsa, Watson and Beach9], do not tend to have a spring peak. However, in New Zealand the spring peak is three times as large as the autumn peak, whereas in England and Wales the spring peak is 20% smaller than the autumn peak [Reference Lake5]. This difference is more pronounced in the South Island where the spring peak is nearly five times the magnitude of the autumn peak. The cryptosporidiosis peak in the South Island (October) also occurs 1 month later than in the North Island (September). This discrepancy is probably due to the increased latitude of the South Island and the consequent delay in the start of the agricultural season (e.g. lambing). The standard deviation bars indicate that between years the cryptosporidiosis rate is proportionally most variable during the autumn (March coefficient of variation=0·9) and less variable in the spring (September coefficient of variation=0·25).
The spring peak might be proportionally larger in New Zealand than in England and Wales for a number of reasons. In both countries the spring peak contains a majority of infections with C. parvum indicating direct or indirect contact with the animal reservoir as the cause of infection [Reference Learmonth1, Reference McLaughlin10]. In the spring, livestock are most infectious due to the birth of large numbers of new, and hence highly infectious livestock. The New Zealand economy is heavily based on dairy, beef and sheep farming [Reference Learmonth1] providing greater opportunities for direct contact with the animal reservoir of infection than in England and Wales. In New Zealand the livestock density is 4017 livestock units per 100 population compared to 1630 in the United Kingdom (FAO statistics). This proposition is supported by the observation that the South Island, with a livestock density greater than the North Island, has a proportionally greater cryptosporidiosis spring peak.
The spring peak may appear proportionally larger in New Zealand due to the small magnitude of the autumn peak. In New Zealand many human samples were C. hominis in the autumn indicating that the source of infection is human in nature. Consequently, incidence may be lower than in England and Wales due the low population density in New Zealand providing fewer opportunities for person-to-person transmission of cryptosporidiosis. In England and Wales the autumn peak is associated with high numbers of imported travel cases and community transmission from these [Reference Lake8]. However, reported travel cases were not significant in any of the autumn regression models suggesting that travel cases are not playing a significant role in the autumn peak in New Zealand.
Turing our attention to the time-series regressions, the Table indicates that between January and May cryptosporidiosis was positively and significantly associated with mean New Zealand temperature in the current and previous month. The autumn cryptosporidiosis peak has been linked to increased recreational water use, swimming, outdoor activities and increased person-to-person spread [Reference Naumova11]. These activities are usually greater in warmer weather [Reference Naumova11] and so the positive associations uncovered between temperature and cryptosporidiosis between January and May suggest the importance of these transmission pathways. The importance of person-to-person transmission is emphasized by the observation that many autumn cases are infected with the C. hominis species [Reference Learmonth1].
Between June and December there were no associations between cryptosporidiosis and weather in New Zealand. This contrasts with England and Wales where strong relationships between cryptosporidiosis in the spring and measures of run-off (river flow) have been found and linked to the ingress of Cryptosporidium into water-courses and drinking-water supplies [Reference Lake5]. It was hypothesized that drinking water would be an important pathway for cryptosporidiosis in New Zealand as in 2005 only 71% of New Zealand's population were supplied with drinking water complying with protozoan regulations [12]. Consequently the lack of association between cryptosporidiosis and river flows or rainfall is unexpected.
River flows will correlate poorly with run-off in New Zealand due to the importance of snow melt to river flows. However, no relationships between cryptosporidiosis and rainfall existed either, although rainfall is also a poor measure of run-off because it will only lead to washing of Cryptosporidium from the land if the soil is already saturated. One further explanation for the lack of a relationship between river flows or precipitation and cryptosporidiosis could be that, in comparison with England and Wales, New Zealand has a number of geographically distinct climatic areas. Consequently, countrywide measures of rainfall or river flow are less appropriate. This proposition was explored by producing separate models for the two district health authorities in New Zealand, Canterbury and Waikato, with the greatest number of cryptosporidiosis cases. Case numbers were then compared to the rainfall and river flow at the same location. However, neither region displayed any consistent associations with weather during the spring. This implies either that water supply is not important to the aetiology of cryptosporidiosis in the spring or that the risk of Cryptosporidium ingress into drinking-water supplies is not associated with rainfall and river flow in New Zealand. There is further support for the latter due to the observation that cryptosporidiosis numbers are relatively stable from year to year in the spring. This may be due to structural differences between the water supply in England and Wales and New Zealand. The latter contains large numbers of small local supplies [12].
ACKNOWLEDGEMENTS
We acknowledge the help of Ruth Pirie at ESR in supplying the cryptosporidiosis health surveillance data and for providing helpful advice about the appropriate use of these data. We also thank Jim Salinger and Kathy Walter of NIWA for data on New Zealand temperature and river flow respectively.
DECLARATION OF INTEREST
None.






