Introduction
Columbidae (pigeons and doves) is a globally represented family of birds yet it has received relatively little conservation attention. Pigeons and doves are represented on every continent except Antarctica and in habitats as diverse as tropical rainforests, deserts, mountains and cities (Goodwin, Reference Goodwin1983; Baptista et al., Reference Baptista, Trail, Horblit, del Hoyo, Elliot and Sargatal1997; Gibbs et al., Reference Gibbs, Barnes and Cox2001). Despite the evolutionary success of the Columbidae, the family contains a significantly greater proportion of threatened species than expected by chance (Bennett & Owens, Reference Bennett and Owens1997), possibly because it is one of a small number of bird families that are prone to human persecution, introduced predators and habitat loss (Owens & Bennett, Reference Owens and Bennett2000).
Columbid diversity is highest in the forests of the tropics where the many frugivorous columbids, through their role as seed dispersers, are ecologically important in the maintenance of tropical forest diversity (Stiles, Reference Stiles, Diamond and Lovejoy1985; Corlett, Reference Corlett1998; McConkey et al., Reference McConkey, Meehan and Drake2004) and in some cases are the only vector by which seeds of certain tree species are dispersed (Meehan et al., Reference Meehan, McConkey and Drake2005). Columbids are also fundamental agents in the regeneration of non-wooded areas and are among the most important agents in the colonization and building of wooded habitats on islands (Whittaker & Jones, Reference Whittaker and Jones1994; Whittaker & Turner, Reference Whittaker and Turner1994; Thornton et al., Reference Thornton, Compton and Wilson1996). As a consequence, local declines in frugivorous columbid populations may have long-term detrimental effects for regeneration of tropical forest ecosystems (Chapman & Chapman, Reference Chapman and Chapman1995; Wright & Duber, Reference Wright and Duber2001). In extreme cases, the loss of columbid species may result in some plants having no means of seed dispersal (Hamann & Curio, Reference Hamann and Curio1999; McConkey & Drake, Reference McConkey, Drake, Levey, Silva and Galetti2002).
In this paper I review the distribution of and threats to Columbidae and identify priority areas for the conservation of pigeon and doves and the forests they maintain.
Methods
I used the data available on the BirdLife International (2007) Bird Information Database, and latest updates supplied by BirdLife International, to examine threats to the Columbidae, and I follow the current columbid taxonomy adopted by BirdLife International. These data provide the information for species accounts used for birds on the IUCN Red List (IUCN, 2006). From the information held on the Bird Information Database I extracted conservation status, distribution (region and country), habitats, threats and data quality. I identified restricted-range species from a data set supplied to me by BirdLife International. Information on the origins of islands inhabited by Columbidae (continental or oceanic) was taken from Stattersfield et al. (Reference Stattersfield, Crosby, Long and Wege1998), and the classification of species as terrestrial or arboreal and data about bird sizes were obtained from Gibbs et al. (Reference Gibbs, Barnes and Cox2001). I collated and analysed these data to examine patterns in extinction risk of Columbidae.
Results
Conservation status
BirdLife International currently recognizes 317 species of columbid, 12 of which are already extinct and one species, the Socorro dove Zenaida graysoni, which is extinct in the wild. Of the 304 species extant in the wild, 19.4% (59) are categorized as Threatened and a further 12.5% (38) as Near Threatened. Overall, nearly one third of extant columbids face some degree of extinction threat. Of the threatened species, 10 (16.9%) are categorized as Critically Endangered, 15 (25.4%) as Endangered and 34 (57.6%) as Vulnerable. Only one species is currently showing signs of recovery, the pink pigeon Streptopelia mayeri, as a result of intense conservation effort. Fifty-two species (88.1%) have populations that are still in decline, although six species (10.3%) have global populations that are stable. The availability and quality of the data available for most threatened species are described as poor (for 24 species) or medium (27 species); good data are available for only eight species.
Geographical distribution
There are three coherent areas of columbid taxonomic distribution: the Americas; Europe, Africa, the Middle East and Central Asia; and Asia, Australasia and Oceania (Fig. 1, Appendix). There are 12 genera and 72 species in the Americas. Nine of these genera (containing 63 species) are endemic to the area (Appendix). The columbids of Europe, Africa, the Middle East and Central Asia are similar as they comprise entirely, or are dominated by, species of Columba and Streptopelia. There are 55 species in this area but with the exception of Africa (47 species), the individual regions have low columbid diversity (Table 1, Appendix). In contrast, there are 196 species of columbid in Asia, Australasia and Oceania, representing 64.5% of the columbid species extant in the wild. There are strong taxonomic affinities within the area: Asia and Australia have 15 genera in common, and Oceania shares four of its five columbid genera with Asia and Australia. These genera are all endemic to these three regions and account for over a third (104) of all extant columbid species (Table 1, Appendix).
Asia, Australasia and Oceania support 75.9% (44) of all threatened species (Fig. 2, Table 1). This is 22.4% of the area’s columbid species, a larger percentage than in the Americas (15.7%, n = 11) or Europe, Africa, the Middle East and Central Asia (9.8%, n = 5). However, this difference is not significant when species representation within each area is controlled (45/196, 11/72 and 5/55, respectively; G = 5.242, df = 2, P = 0.091).
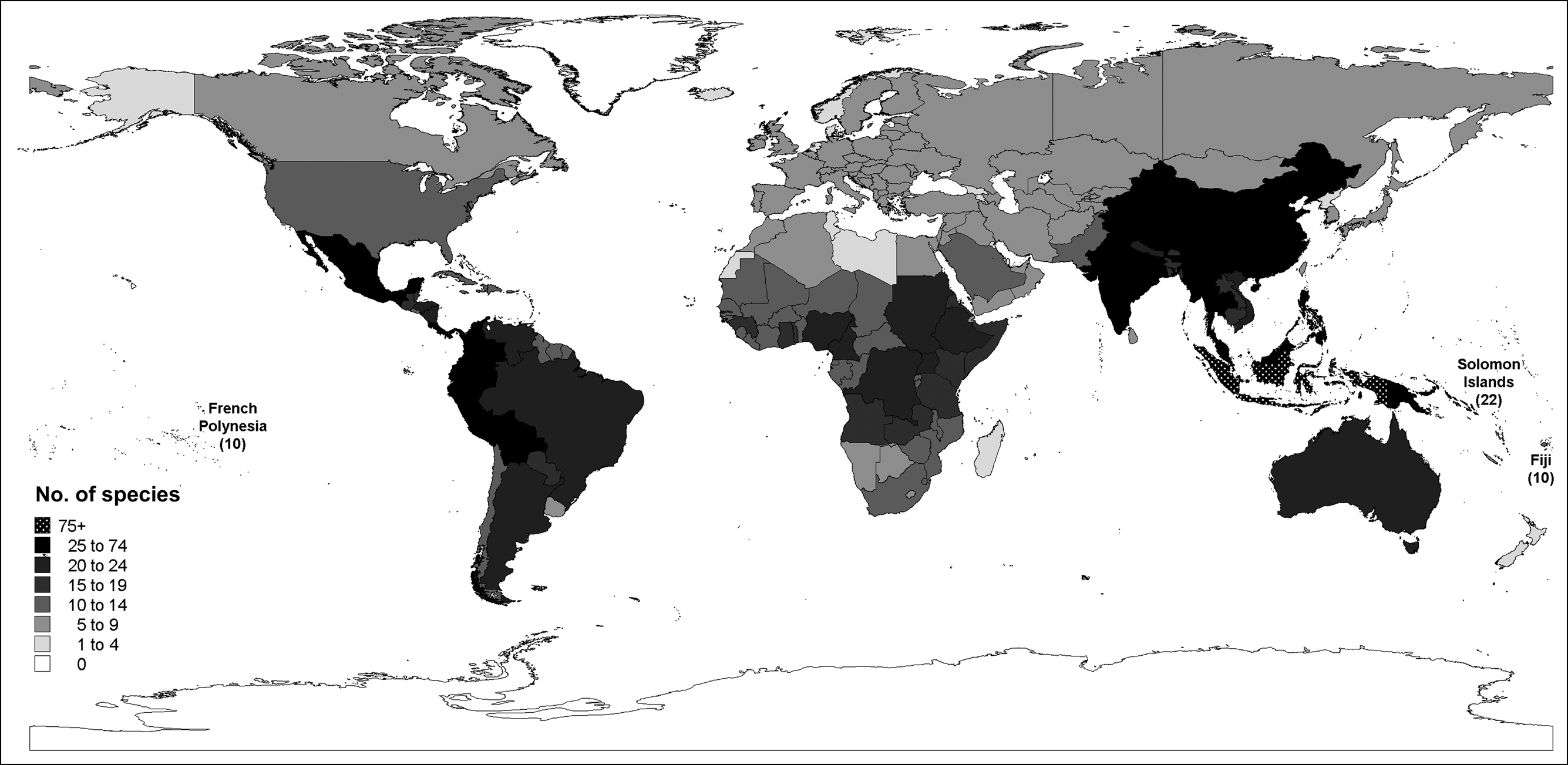
Fig. 1 Number of columbid species in each country, including extinct species, with the number of species presented directly for selected islands. Species from remote territories are not attributed to mainland totals; for example, species on the Canary Islands are not included in the count for mainland Spain.
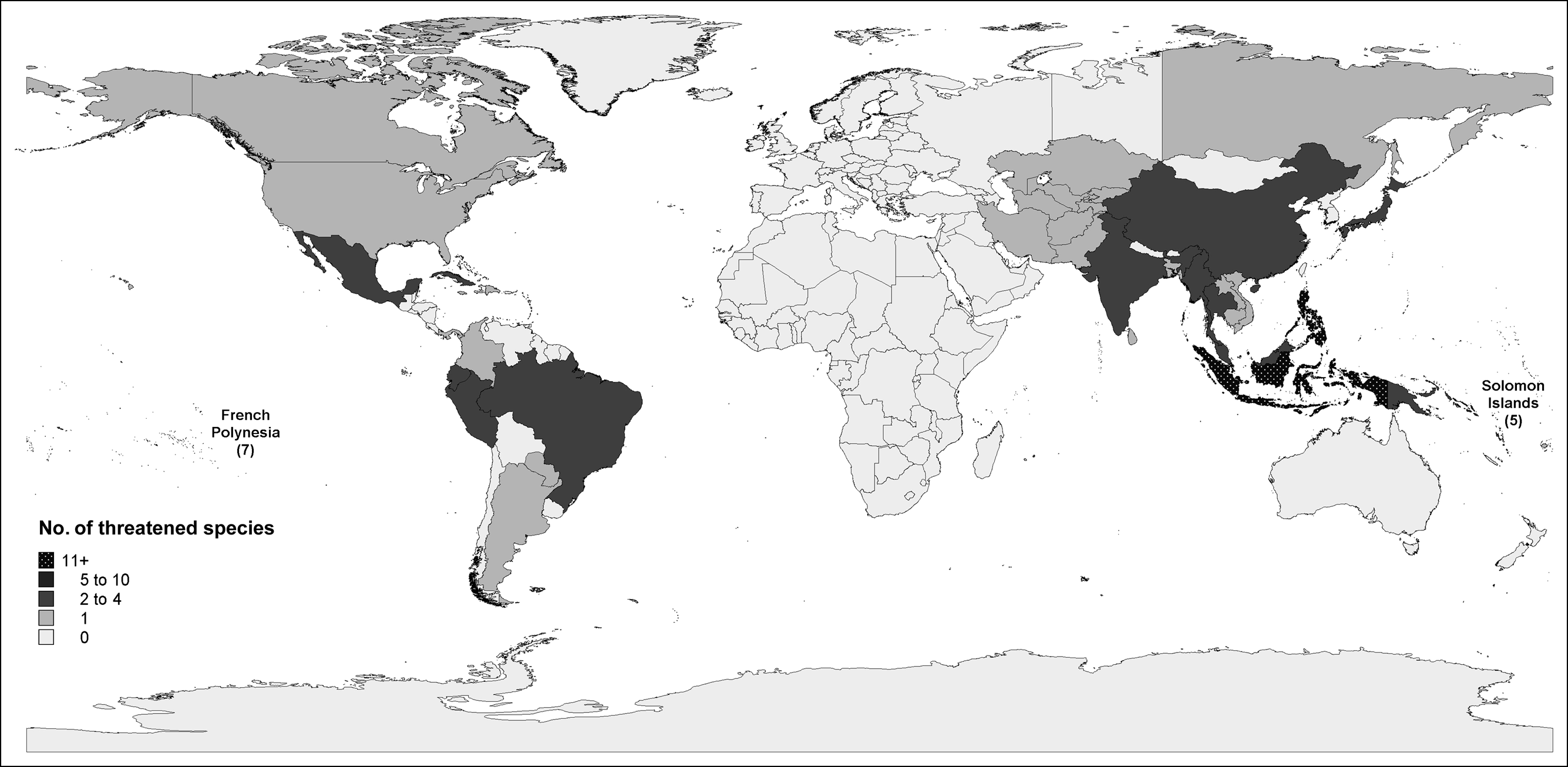
Fig. 2 Number of threatened (i.e. Critically Endangered, Endangered and Vulnerable) columbid species in each country, with the number of species presented directly for islands that have ≥5 threatened species. Threatened species on remote territories are not attributed to mainland totals; for example, threatened species on the Canary Islands are not included in the threatened species count for mainland Spain.
Table 1 Numbers of Extinct, Threatened and Near Threatened columbid species in the Americas, Europe, Africa, the Middle East and Central Asia, and Asia, Australia and Oceania, and in regions within these three areas.
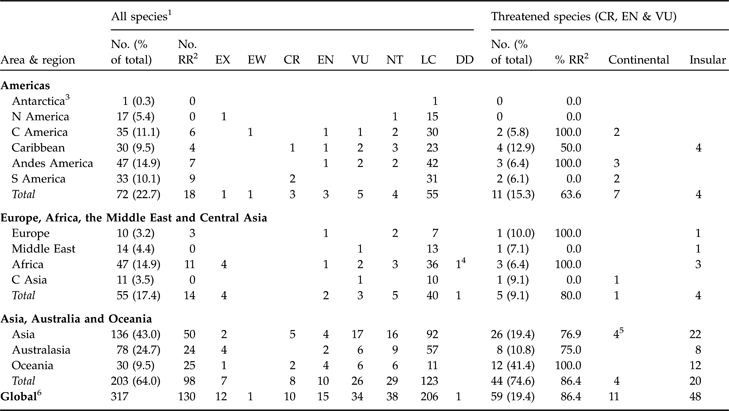
1 EX, Extinct; EW, Extinct in the wild; CR, Critically Endangered; EN, Endangered; VU, Vulnerable; NT, Near Threatened, LC, Least Concern; DD, Data Deficient
2 With restricted range
3 Eared dove Zenaida auriculata occurs on South Georgia and the South Sandwich Islands
4 Insufficient data are available to assess accurately the threat status of the Somali pigeon Columba oliviae.
5 Large green pigeon Treron capellei is both a continental and insular species, and treated here as continental.
6 11 species occur in more than one area: 9 occur in Asia, Australasia and Oceania and Europe, Africa, the Middle East and Central Asia; and 2 occur in all three areas.
Asia, Australasia and Oceania is the area of greatest importance in the conservation of columbid diversity. Within this area the region with the highest columbid diversity is Asia, containing 44.1% (134 species) of all extant species and 44.8% of all threatened species. Australia is the region with the second richest columbid diversity, containing 24.7% of species (75) and 13.6% of threatened species. Oceania supports just 9.5% of extant species (29) but with 12 (41.4%) of these species listed as threatened, a fifth of all threatened species occur there.
In terms of the numbers of threatened species, certain countries are particularly important for columbid conservation. Of the eight countries with three or more threatened species, all are either in Asia, Australasia or Oceania (Table 2). Indonesia has 93 species, more than any other country and nearly a third of the world’s species. All Indonesian species are still extant, although 12.9% (12) are currently threatened. The Philippines has 11 threatened species, which is nearly one-third of its 34 columbid species (all extant). In French Polynesia, two-thirds (6/9) of extant species currently face extinction, the greatest proportion of any country.
Table 2 The number of Extinct, threatened (Critically Endangered, Endangered, Vulnerable), Near Threatened and Least Concern species of Columbidae in countries with three or more threatened species (with % of total species in each country in parentheses).
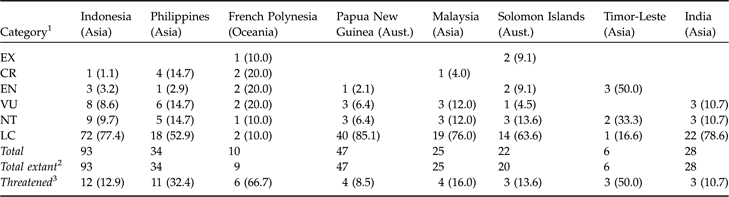
1 See footnote to Table 1
2 Extant in the wild
3 Expressed as number of species (% of total number of extant species in each country)
Range sizes
There are more restricted range species in Asia, Australasia and Oceania (98/196, 50.0% of species) than in the Americas (18/72, 25.0%) or Europe, Africa, the Middle East and Central Asia (14/55, 25.5%; G adj = 20.32, df = 2, P = 0.000). Forty-nine (83.1%) of threatened species have restricted ranges and all but six of these are insular species (one species, the large green pigeon Treron capellei, is both a continental and insular species, and for the purposes of this study is treated as continental; Table 1). Restricted range insular species have a significantly greater representation among threatened species than expected (21.2 species) from the global numbers of extant restricted-range insular species (109/304; G adj = 33.31, df = 1, P = 0.000). Asia, Australasia and Oceania supports 37 of these threatened restricted-range insular species, 86.6% of the regions’ threatened species and significantly more than expected (22 species, calculated from the proportion of restricted range insular species in these regions; G adj = 24.20, df = 1, P = 0.000). Seven of the 11 threatened species with continental distributions are from South and Central America and represent all the threatened species within these two regions.
Altitudinal range
Threatened columbids have narrower altitudinal ranges (mean 1,094 ± SD 627 m, n = 48) than columbids of Least Concern (mean 1,652 ± SD 831 m, n = 180; t =1.971, df = 175.4, P = 0.050), because despite their having significantly lower minimum altitudinal extents (mean 112 ± SD 274 m, n = 57 and mean 213 ± SD 510 m, n = 193 respectively; t = 1.971, df = 175.4, P = 0.000), the maximum elevations of their ranges are also significantly lower (mean 1,232 ± SD 688 m, n = 47) than those of columbids of Least Concern (mean 1,881 ± SD 1,022 m, n = 180; t = 5.158, df = 105.3, P = 0.000).
Habitats
All threatened columbids are subtropical/tropical species with the sole exception of the pale-backed pigeon Columba eversmanni, which is a winter visitor to the subtropics/tropics from its breeding grounds in arable lands further north. Of the subtropical/tropical columbids, all inhabit forest except for the blue-eyed ground dove Columbina cyanopis, which is a grassland species. Forests are classified as either a critical or major habitat for 56 threatened species (94.9%).
Status of terrestrial versus arboreal columbids
Terrestrial columbids account for 38.2% (116/304) of extant species and are better represented in the Americas (51, 75.0% of species) than in Europe, Africa, the Middle East and Central Asia (17, 42.5% of species) and Asia, Australasia and Oceania (43, 23.2% of species; G =57.17, df = 2, P = 0.000). Terrestrial columbids account for 40.7% (24) of threatened species and are neither over nor under represented in relation to their global species representation (G adj = 2.27, df = 1, P = 0.165). There are more threatened terrestrial species in Asia, Australasia and Oceania (14) than in either the Americas (9) or Europe, Africa, the Middle East and Central Asia (1). However, neither terrestrial (nor arboreal) species are over or under represented among threatened species in the Americas (obs. = 9, exp. = 8.25; G adj = 0.280, df =1, P = 0.597), Europe, Africa, the Middle East and Central Asia (obs. = 1, exp. = 2.55; G adj = 1.697, df = 1, P = 0.193) or Asia, Australasia and Oceania (obs. = 14, exp. = 9.98, G adj = 1.93, df = 1, P = 0.165).
Threats
The threats cited as reasons for categorizing columbids as at risk from extinction are presented in Table 3. There are three major threats to columbids: habitat loss, hunting and invasive alien predators. Habitat loss/degradation is listed as a pressure to all threatened species except the Marquesan ground-dove Gallicolumba rubescens, which is at risk solely as a result of alien predators. The type or cause of habitat loss/degradation is identified for 49 species. Agriculture affects most of these (45, 91.8%) followed by either wood extraction or mining (38, 77.6%). Over half of the threatened species are affected by both agriculture and extraction (32, 54.2%) whereas just eight species (13.6%) are threatened by a single type of pressure on their habitat. The level of forest fragmentation within species’ ranges has been qualified for 56 threatened species. Of these, only five species (8.9%) have experienced no forest fragmentation, 36 species (64.3%) have experienced some and 15 species (26.8%) have experienced severe fragmentation.
Table 3 The threats implicated in the extinction risks of Columbidae. Values are number of species (% of species within each threat area or class).
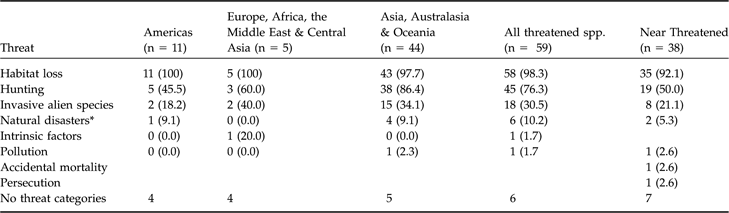
* Storms/flooding
Hunting affects just over three-quarters of threatened species (45). Most (41) are hunted as a source of food, with this pressure more prevalent among species in Asia, Australasia and Oceania than in the Americas or Europe, Africa, the Middle East and Central Asia (G adj = 9.40, df = 2, P = 0.009). This may reflect their relative attractiveness as a source of bushmeat, for example, because columbid species in Americas are c. 17% smaller (mean wing length = 149 ± SD 40.6 mm, n = 67) than species in Europe, Africa, the Middle East and Central Asia (mean 182 ± SD 45.8 mm, n = 40) and Asia, Australasia and Oceania (mean 179 ± SD 53.8 mm, n = 186; F 2,290 = 9.751, P = 0.000). No more terrestrial than arboreal species are threatened as a result of being hunted for food than expected (G adj = 1.407, df = 1, P = 0.236). Nine species (15.5%), all Asian, are threatened by the cage bird trade, seven are hunted for sport, and the three crowned pigeons Goura spp. are hunted for cultural activities (Table 3).
Invasive alien predators are a threat to 19 species (32.8%). Four of these species also suffer the additional threat of introduced pathogens/parasites and competitors. Alien species are only listed as a threat to insular species, predominantly oceanic island species (18 out of 19 species). Terrestrial and arboreal species are equally threatened by invasive alien predators (G adj = 0.951, df = 1, P = 0.329).
Future threatened columbids
Of the 38 Near Threatened columbid species all but one inhabits subtropical/tropical forest. Habitat loss is the reason for the categorization of 92.1% of these species, hunting for 50.0% of species, and both are reasons for the categorization of 47.0% of Near Threatened species. Eight species are Near Threatened partially or solely because of introduced species, seven of which are island species (Table 3). Asia, Australasia and Oceania are home to over three-quarters (29) of Near Threatened species. Two-thirds (23) of these are restricted-range insular species; significantly more than expected for the area (exp. = 13.9 species, calculated from the proportion of species in these regions that are restricted-range island species, 94/196; G adj = 11.885, df = 1, P = 0.0005). One-third (12) of these species only occur in Asia, which supports 16 (42.1%) of all Near Threatened species. Indonesia supports nine of these species and the Philippines five species. Combined, these two countries support over a third (13) of all Near Threatened species.
Discussion
Patterns of threat
Fifty-nine species of Columbidae, 19% of all species, are currently threatened with extinction. These are virtually all confined to the tropics, are dependent on forests, and predominantly inhabit islands and have restricted ranges. Over three-quarters of all threatened columbid species live in the tropical forests of Asia, Australasia and Oceania, where 91% inhabit islands, 86% have restricted ranges, and 64% are insular species with restricted ranges. Small geographic ranges and endemism have previously been identified to be significantly associated with extinction risk among columbid species (Hughes, Reference Hughes2004), and reliance on rare food resources has been identified as a correlate of their local rarity (Walker, Reference Walker2006). Countries or territories within Asia, Australasia, and Oceania are the most important for the conservation of columbid diversity. The islands of the Indonesian and Philippine archipelagos and the islands of French Polynesia are of exceptional importance as together they support 40% of all extant species of columbid and half of all threatened species.
Threats
The threats affecting most species of threatened columbids are habitat loss and degradation, hunting, and invasive alien species.
Habitat loss Habitat loss is the greatest threat facing columbid diversity. It is listed as a threat to all but one threatened species. Halting, and for some species reversing, forest loss, fragmentation and degradation is essential to the global conservation of threatened pigeons and doves. The Americas and Africa have greater rates of deforestation than Asia, Australasia and Oceania (FAO, 2005) and this is currently the greatest cause of threat in these areas. Forest clearance has been high within Asian countries in the past. For example, Indonesia, which has the greatest columbid diversity and the most threatened columbids, has lost 40% of its forests since 1950 (FWI/GFW, 2002). That so many threatened species occur on islands within Asia, Australasia and Oceania probably reflects the fact that even the lower contemporaneous rates of clearance on small tropical islands significantly reduces overall forest cover.
It is not just the extent, but also the quality of remaining forest that affects columbids. Many species have been identified as being affected by fragmentation because they display minimum thresholds in the size of the forest they inhabit or visit (Warburton, Reference Warburton, Laurance and Bierregaard1997; Jati, Reference Jati1998; Price et al., Reference Price, Woinarski and Robinson1999). Different species are also affected by different habitat qualities (Marsden, Reference Marsden1998; Kavanagh & Stanton, Reference Kavanagh and Stanton2005; Peh et al., Reference Peh, de Jong, Sodhi, Lim and Yap2005). Threatened columbid species, however, tend to favour primary forest (Jones et al., Reference Jones, Linsley and Marsden1995; Peh et al., Reference Peh, de Jong, Sodhi, Lim and Yap2005) and are therefore negatively affected by forest degradation.
Hunting Hunting is a greater threat to species at risk of extinction in Asia, Australasia and Oceania than in the rest of the world. This may be because on the islands that make up these regions agricultural protein production may not meet demand or bushmeat may be a preferred source of protein. Columbids may be targeted as there may be fewer or less attractive, sources of bushmeat than in the rest of the world (columbids are larger and therefore more profitable to hunt in Asia, Australasia and Oceania). In these areas, if hunting columbids is for a source of protein, then there need to be sustainable agricultural development initiatives that increase existing protein production, or provide alternative protein sources. If hunting is to obtain cash from their trade then sustainable development initiatives that provide community members with an alternative source of income are needed.
Quantification of harvesting levels is difficult to determine. One indication is the level of trade in species. CITES monitors international trade in controlled columbid species. Five species are listed on CITES Appendices I - III (CITES, 2007); all were listed at the start of CITES in either 1975/1976. No illegal trade has been recorded for any species currently listed on CITES Appendices since 1989 (calculated from CITES, 2007), indicating that illegal international trade does not represent a significant threat to CITES-listed species.
CITES regulations do not regulate the international trade in non-CITES species, which is known to exist although is rarely quantified. Surveys of trade in non-CITES bird species in South-east Asia (which included an Appendix III species that was not widely recognized in the region) identified Indonesia to be the most active trader in its own wildlife in South-east Asia (Nash, Reference Nash1993). Trade in 32 non-CITES wild columbids in South-east Asia was recorded, with a significant export trade in fruit doves Ptilinopus spp. and imperial pigeons Ducula spp., particularly from West Papua.
National trade is harder to monitor. In Indonesia Shepherd et al. (Reference Shepherd, Sukumaran and Wich2004) monitored the live bird trade in Medan, Sumatra, a major centre in the domestic and international trade of wildlife, between 1997 and 2001. They recorded 17 identified and five unidentified species of columbid for sale as meat, songbirds, pets, or for ceremonial/medicinal purposes. The traded species included both Near Threatened and globally threatened species. Not all hunted columbids make it to formal markets, and unknown quantities are sold by roadside or opportunistic traders or are for personal use/ consumption. Hunting of rare columbid species is legal in some countries (BirdLife International, 2005a) and records obtained opportunistically show that capture and informal trade of Globally Threatened species still takes place (BirdLife International, 2005b). Although numbers of birds captured for personal use and local domestic trade are completely unknown, given that hunting, mostly for subsistence food, is listed as a threat to 76% of threatened and 50% of Near Threatened columbid species it is likely to continue to represent a significant pressure on many columbid populations.
Introduced alien species Eighteen species are listed as threatened from introduced alien species (predators, competitors, and pathogens/parasites). Such species are currently only a threat to species that inhabit islands. Introduced predators that have resulted in columbid declines are most commonly rats Rattus spp. and feral cats Felis catus, but include crab-eating macaques Macaca fascicularis, possums Trichosurus vulpecula, swamp harriers Circus approximans, and mongooses Herpestes auropunctatus (Clout et al., Reference Clout, Karl, Pierce and Robertson1995; Gibbs et al., Reference Gibbs, Barnes and Cox2001; Powlesland et al., Reference Powlesland, Wills, August and Augus2003). The destruction or degradation of columbid habitat from introduced/invasive species is also a problem. For example, feral goats and pigs have destroyed large patches of the natural forest habitat of the Marquesan imperial pigeon, an arboreal species restricted to the French Polynesian island of Nuku Hiva, and was the cause of the threat categorization of this species (Villard et al., 2003). To assess and address the influence of alien species on columbids requires the identification of their roles (competitors, predators, modifiers of habitat) and effects on columbid food supply, mortality levels and nesting success. The control of alien species with negative impacts on columbids, however, will depend on the species in question.
Future research and priorities
Good quality data are only available for 14% of threatened species. This is a great hindrance to halting and reversing population declines. To manage wild populations effectively and to identify and manage species that may require ex situ conservation efforts, research is needed to determine species’ distributions and population sizes and identify species’ resource requirements and reproductive output.
Most threatened columbids are tropical forest species but these are notoriously difficult to observe and study. For example, columbid calls are often superficially similar and identification of a species’ full repertoire can be time consuming. Reliance on just the most common contact calls of species, however, will lead to lost potential from fieldwork and possible under-recording of species in studies of population sizes. Such problems can lead to information degradation in both data collection and analyses as species are sometimes lumped together, possibly because of ambiguous identification or because insufficient species-specific data are collected (Marsden, Reference Marsden1998). Effective and efficient methods to collect and analyse data need to be developed and shared amongst both researchers and managers.
Although such data are still required for most threatened species, urgent conservation attention needs to be focused on the Philippines and French Polynesia where a great proportion of columbid species are already threatened or Near Threatened, and where species are likely to become Near Threatened or threatened in the future. In addition, Indonesia should receive attention given the country’s continued deforestation and the high number of columbid species that could become threatened should this trend continue. One particular genus of terrestrial doves, Gallicolumba, requires urgent research and conservation attention. Of the 20 species in this genus, three species are already extinct, 10 are threatened with extinction (17% of the world’s threatened species) and three are Near Threatened. Twelve (71%) of these species occur in Indonesia, the Philippines or French Polynesia.
A few pigeon species have been the subjects of autecological research and successful conservation action; for example, captive breeding programmes have brought two species of pigeon back from the brink of extinction, the pink pigeon and Socorro dove (Butchart et al., Reference Butchart, Stattersfield and Collar2006). In New Zealand there is considerable interest and commitment to conserving the country’s only native species of pigeon, the Near Threatened New Zealand pigeon Hemiphaga novaeseelandiae, which has suffered rapid declines in recent years. Several projects are now working to highlight the plight of the species and secure its long-term future. Such timely awareness, and conservation commitment and resources, need to be generated and targeted towards the many threatened pigeon and dove species in the tropics.
Acknowledgements
I would like to thank Stuart Butchart (BirdLife International), Elizabeth Mutch, Noorainie (TRAFFIC), Sue Shutes (BirdLife International), David Orchard and two anonymous reviewers.
Appendix
Distribution of columbid genera and species following the taxonomy and geographic divisions adopted by BirdLife International (2007). Vagrants and introduced species are not included.
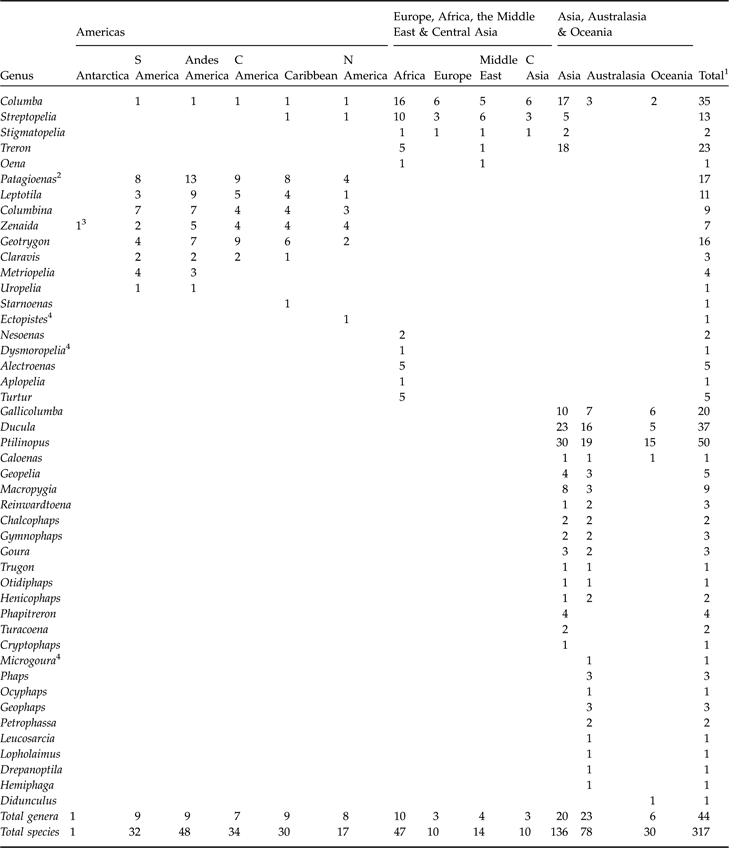
1 Represents the total number of species within each genus, not the row total, which is sometimes higher because species can occur in more than one region.
2 Formed from splitting Columba in 2003
3 Eared dove Zenaida auriculata occurs on South Georgia and the South Sandwich Islands
4 Extinct genus
Biographical sketch
Jonathan Walker has studied frugivorous pigeon feeding ecology in the tropical forests of Indonesia for several years and has long championed the conservation of columbids (see http://www.columbidae.org.uk). His major research interests include extinction risk in birds, frugivory, and sustainable agriculture.










