Globally the population is ageing, with predictions that the number of people aged 60 years and over will reach up to two billion by 2050(1). An estimated 23 % of the global burden of disease arises in older people, and mental disorders are reported as the leading cause of disability and ill health(Reference Prince, Wu and Guo2). Dementia and depression are the most common of these disorders in ageing as identified by the WHO(3). Cognitive function declines with age, ranging in severity from mild cognitive impairment (MCI) to dementia, with up to 50 % of those with MCI going on to develop dementia within 5 years(Reference Gauthier, Reisberg and Zaudig4). MCI can be defined as cognitive decline greater than expected for an individual's age and education level but that does not interfere notably with activities of daily life(Reference Gauthier, Reisberg and Zaudig4), whereas dementia interferes with activities of daily living(5). Dementia currently affects 46·8 million people worldwide and is projected to affect over 131 million people by 2050(Reference Prince, Comas-Herrera and Knapp6), while depression is anticipated to be the second leading cause of disability worldwide by 2020(7), with 22 % of males and 28 % of females over the age of 65 years affected by depression(Reference Craig, Mindell and Becker8). The economic burden of cognitive decline and depression is profound. Experts have calculated that dementia will be a trillion dollar disease by 2018(Reference Prince, Comas-Herrera and Knapp6). Figures for depression are currently estimated at over €3 billion in Ireland(Reference O'Shea and Kennelly9) and £7·5 billion in England(7). With mental health considered to be one of the greatest global challenges(Reference Livingston, Sommerlad and Orgeta10), there is an urgent need to identify modifiable factors for targeted interventions to promote better brain health in our ageing populations. Epidemiological evidence supports a role for certain dietary factors in brain health, opening up new potential avenues for prevention of dementia and mental illness in ageing(Reference Panza, Solfrizzi and Capurso11, Reference Rechenberg12).
This review will explore the influence of ageing on brain health and the emerging evidence linking diet and specific nutrients with cognitive function and depression in ageing. The use of novel imaging technologies in nutrition and brain research will be discussed, along with the potential for nutrition to play a protective role in preserving better brain health in ageing.
The ageing brain
Physiology and pathophysiology
The structure and metabolic pathways within the brain are progressively altered with ageing, although the precise aetiologies of ageing have not been fully elucidated. As people age, there is a reduction in brain volume in both grey and white matter(Reference Resnick, Pham and Kraut13), while white matter lesions increase(Reference Peters14) and there is development of amyloid plaques, neurofibrillary tangles, Lewy bodies, synaptic dystrophy and neuron loss(Reference Svennerholm, Jungbjer and Boström15, Reference Elobeid, Libard and Alafuzoff16), which have been suggested to parallel the progression of cognitive decline(Reference Serrano-Pozo, Frosch and Masliah17). There are also changes in the production of neurotransmitters, in particular serotonin and dopamine, which have been reported to decline by up to 10 % per decade from early adulthood(Reference Peters14). Additionally, there is an increase in oxidative stress response(Reference Bishop, Lu and Yankner18) and more dysfunction of the blood–brain barrier(Reference Goodall, Wang and Simpson19).
Normal ageing is associated with a decline in cognitive function, with most cognitive change observed in memory during the ageing process. MCI is a recognised clinical condition where individuals have evidence of cognitive impairment but do not meet the criteria for the diagnosis of dementia(Reference Winblad, Jelic and Wahlund20). Alzheimer's disease (AD) is the most common form of dementia, accounting for 62 % of cases, with other forms including vascular dementia, mixed, Lewy body and frontotemporal dementia(Reference Prince, Knapp and Guerchet21). Depression in older adults is often referred to as late-life depression and is reported more commonly in females than males(Reference Luppa, Sikorski and Luck22–Reference Büchtemann, Luppa and Bramesfeld24). The depressive symptoms of older adults are thought to be different from those experienced by younger adults, as somatic and psychological symptoms are often accompanied by fatigue, hopelessness about the future, loss of appetite and sleep disturbance(Reference Luppa, Sikorski and Luck22).
Pharmaceutical treatments
Pharmacological treatment for dementia is prescribed by specialist clinicians(25), but only a limited number of medications that target the biochemical abnormalities of neuronal loss are included within the National Institute for Health and Care Excellence recommendations for dementia interventions. These include acetylcholinesterase inhibitors (donepezil, galantamine, rivastigmine) and memantine (N-methyl-d-aspartate receptor antagonists). There are however a variety of pharmacological treatment options available for depression including tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin and noradrenaline reuptake inhibitors and selective noradrenaline reuptake inhibitors(Reference Rang, Ritter, Flower, Rang, Dale, Ritter, Flower and Henderson26, Reference Mann27). Overall, poor response rates to these costly pharmacological treatments for depression have been observed(Reference Ilyas and Moncrieff28, Reference Rush, Trivedi and Wisniewski29), and despite significant investigation into the role of pharmacological treatments for dementia, no licenced medication can cure these diseases of the brain. Therefore, much effort is currently focusing on options for prevention rather than treatment of brain disorders.
Assessment of brain function
The assessment of brain function for neurodegenerative diseases and depressive disorders in ageing is a developing area. There are numerous neurological tests available which are designed to assess and distinguish different individuals in their response to day-to-day cognitive tasks(Reference de Jager, Dye and de Bruin30) and for the detection of common mental health disorders(Reference Ali, Ryan and De Silva31). The National Institute for Health and Care Excellence has provided guidance on the recommended diagnostic criteria for depression(32) and dementia(33). For dementia, the guidelines emphasise the need to assess the following domains: attention and concentration, orientation, short- and long-term memory praxis, language and executive function. Furthermore, the National Institute for Health and Care Excellence recommends that formal tests should be conducted, including the mini mental state examination, six-item cognitive impairment test, general practitioner assessment of cognition and 7-min screen, and that other factors known to influence performance such as education level, should also be taken into account. Lastly, only healthcare professionals with expertise in differential diagnosis and using international standardised criteria (such as the National Institute of Neurological Communicative Disorders) should be responsible for diagnosing subtypes of dementia(33).
Investigating cognitive and mental health outcomes via questionnaire-based assessments is the most common approach for assessing the effects of nutrition(Reference Macready, Butler and Kennedy34). For assessing brain health and function in relation to nutritional factors, studies should be aimed at prevention rather than treatment, and non-nutrition factors contributing to cognitive impairment and depression should be incorporated into studies and considered at the time of analysis(Reference de Jager and Kovatcheva35). Concerning the specific tests to assess cognitive function, these should be carefully selected and should be based on a known or hypothesised relationship of a specific food/nutrient with cognitive function and not solely on their availability or ease of administration. It is also important that the tests are suitable for repeated administration, are appropriate to the population being studied and are relatively simple to interpret and administer. More work is required using standardised tests across laboratories, so that the specific tests or markers that are most sensitive to the nutrients tested can be established(Reference de Jager, Dye and de Bruin30, Reference de Jager and Kovatcheva35). Lastly, computerised cognitive assessments have been utilised and these should be considered for use in future trials in terms of their accuracy and ability to capture reaction-time data, standardisation of administration, availability of parallel versions of tasks for testing at multiple time points and availability in multiple languages(Reference de Jager and Kovatcheva35).
Food, nutrition and brain health in ageing
Foods and dietary patterns
Increasing evidence implicates certain dietary patterns such as higher intake of fruit and vegetables(Reference Kang, Ascherio and Grodstein36) and fish(Reference Barberger-Gateau, Raffaitin and Letenneur37) as being beneficial to brain health. The Mediterranean diet is receiving significant attention as regards its role in preserving cognitive health and protecting against depression in ageing. This diet is typically characterised by higher intakes of fruit, vegetables, wholegrains, fish, unsaturated fatty acids and a regular but moderate consumption of alcohol. A recent meta-analysis (n 34 168) showed that the highest Mediterranean diet score was associated with reduced incidence of developing cognitive disorders (RR 0·79, 95 % CI 0·70, 0·90)(Reference Wu and Sun38) while supplementation of the Mediterranean diet with olive oil or nuts was associated with improved cognitive function(Reference Valls-Pedret, Sala-Vila and Serra-Mir39). Of note, studies using MRI have shown that adherence to the Mediterranean diet was associated with larger cortical thickness (which in turn is associated with a lower risk of cognitive impairment)(Reference Staubo, Mielke and Petersen40). There is also accumulating evidence to support a potential role for the Mediterranean diet in preventing depression in older adults, with cross-sectional and prospective studies showing inverse associations between Mediterranean diet score and risk of depression(Reference Psaltopoulou, Sergentanis and Panagiotakos41–Reference Veronese, Stubbs and Noale45). Further well-designed intervention studies are however required to more fully investigate the potential role of the Mediterranean diet as a means of helping to preserve better brain health in ageing.
Specific nutrients
Protein and carbohydrates
The role of dietary protein intake on cognitive function or mental health has not been extensively studied in ageing populations. Lower verbal memory scores were however observed in older people with lower dietary protein intakes(Reference Goodwin, Goodwin and Garry46). Additionally, higher dietary protein intake was found to be positively correlated with non-verbal learning, verbal memory and reduced risk of MCI or dementia(Reference Roberts, Roberts and Geda47, Reference Koehler, La Rue and Wayne48). One randomised controlled trial (RCT) investigating the effects of dietary protein from red meat on cognitive function in older adults is in progress (ACTRN12613001153707) with results expected in 2018(Reference Daly, Gianoudis and Prosser49).
The association between carbohydrates and cognitive function is unclear because available evidence is scarce, with one Cochrane review identifying only one relevant RCT in older adults(Reference Ooi, Loke and Yassin50, Reference Power, O'Connor and Ross51). However, higher dietary carbohydrate and sugar intakes were associated with lower cortical thickness, which is in turn associated with high risk of late-life MCI and dementia(Reference Staubo, Mielke and Petersen40). While more research has focused on carbohydrates and depression, the available evidence is somewhat conflicting. One study of community-dwelling older adults found that those with depressive symptoms consumed a diet with a higher glycaemic index and glycaemic load(Reference Mwamburi, Liebson and Folstein52). A prospective investigation also reported that a high glycaemic index diet was associated with an increased risk of depression(Reference Gangwisch, Hale and Garcia53). Contrary to these findings, however, institutionalised older adults with depression were reported to consume diets with a lower glycaemic load (Reference Aparicio, Robles and López-Sobaler54). Given the inconsistencies in this area, there is clearly a need for further well-designed studies.
n-3 Fatty acids
The fatty acid composition of the brain membrane is directly affected by diet and this has focused attention on the role of dietary fatty acids in brain health. There is evidence that long-chain n-3 PUFA, EPA and DHA, have potential benefits in cognitive and mental health(Reference Grosso, Galvano and Marventano55, Reference Gillette-Guyonnet, Secher and Vellas56). One meta-analysis of ten randomised trials concluded that n-3 fatty acids may have a protective effect on certain cognitive domains in cognitively impaired patients, however, no effects were seen in healthy people or in AD sufferers(Reference Mazereeuw, Lanctot and Chau57). A recent Cochrane review, which identified three randomised trials for inclusion involving 632 patients with mild to moderate AD, concluded that there was no convincing evidence that PUFA had a role in the treatment of people with existing dementia(Reference Burckhardt, Herke and Wustmann58).
Conversely, systematic reviews and meta-analyses of randomised trials have reported significant clinical benefits of n-3 PUFA intervention in the treatment of depression. The use of predominantly EPA compared with DHA supplementation appears to have greater efficacy(Reference Hallahan, Ryan and Davis59, Reference Grosso, Pajak and Marventano60). Furthermore, supplementation with EPA-predominant formulas as an adjuvant therapy to antidepressants was found to have greater clinical efficacy in the treatment of depression (compared with antidepressants alone), but did not prevent depressive symptoms among populations without a diagnosis of depression(Reference Hallahan, Ryan and Davis59, Reference Grosso, Pajak and Marventano60). A Cochrane review in this area reported a small to modest non-clinical beneficial effect of n-3 PUFA in depression symptomology, but concluded that there was not enough good quality evidence to determine the effect on depression(Reference Appleton, Sallis and Perry61).
Polyphenols
The role of these phytochemicals in brain health and ageing is an emerging area(Reference Schaffer, Asseburg and Kuntz62–Reference Brickman, Khan and Provenzano64). Large prospective studies have identified associations between the dietary intakes of total or specific polyphenols and cognitive function after up to 13 years of follow-up investigation(Reference Letenneur, Proust-Lima and Le Gouge65–Reference Rabassa, Cherubini and Zamora-Ros67). Supplementation with cocoa flavanol for periods of up to 2 months was reported to improve cognitive performance in a group of cognitively intact older adults(Reference Mastroiacovo, Raffaele and Pistacchio68). Of note, Brickman et al. (Reference Brickman, Khan and Provenzano64) conducted a 3-month intervention and showed significant increases in cerebral blood volume in the dentate gyrus as measured by functional MRI in subjects who were assigned to a high flavanol treatment. Research into the role of polyphenols in depression in human subjects has been limited(Reference Pase, Scholey and Pipingas69), although animal studies show promise in demonstrating antidepressant-like effects of polyphenols in mouse models(Reference Zhu, Shi and Wei70).
Vitamins
Specific vitamins have been investigated in relation to brain health and disease. Oxidative stress is thought to be a major contributor to neurodegeneration and depression(Reference Bishop, Lu and Yankner18), thus antioxidants have received much interest. The roles of vitamin C(Reference Hamer, Bates and Mishra71–Reference Luchsinger, Tang and Shea74), β-carotene(Reference Tsuboi, Hori and Kobayashi75–Reference Engelhart, Geerlings and Ruitenberg77) and vitamin E(Reference Banikazemi, Safarian and Mazidi78–Reference Morris, Evans and Bienias81) have been explored, but no clear conclusions can be made and further work in the form of intervention studies is warranted. The postulated roles of vitamin D and B-vitamins have been more fully investigated in relation to their effects on brain health in ageing.
Following the discovery of the vitamin D receptor in the brain(Reference Eyles, Smith and Kinobe82), evidence for the role of vitamin D in brain health has been accumulating. Systematic reviews and meta-analyses have shown that AD sufferers have lower serum vitamin D status than healthy controls, and that low serum vitamin D status is associated with worse cognitive outcomes(Reference Annweiler, Llewellyn and Beauchet83–Reference van der Schaft, Koek and Dijkstra85). Recent longitudinal studies with mean follow-up periods of over 4 years found that lower vitamin D status was also associated with declining mini mental state examination scores and accelerated cognitive decline(Reference Miller, Harvey and Beckett86, Reference Toffanello, Coin and Perissinotto87). Furthermore, Hooshmand et al. used MRI to demonstrate that higher vitamin D status was associated with greater brain volumes(Reference Hooshmand, Lökk and Solomon88), which is generally regarded as a valid marker of disease state and progression. Research investigating the role of vitamin D in depression is much less clear. Large cross-sectional and prospective studies reported that lower serum vitamin D status was associated with an increased risk of depression(Reference Williams, Sink and Tooze89, Reference Brouwer-Brolsma, Dhonukshe-Rutten and van Wijngaarden90). One detailed systematic review, which included cross-sectional, prospective and RCT data, concluded that lower vitamin D status may be a risk factor for late-life depression(Reference Okereke and Singh91).
One-carbon metabolism and related B-vitamins
Historically, B-vitamin deficiencies, in particular folate(Reference Carney92, Reference Reynolds, Preece and Bailey93) and vitamin B12(Reference Strachan and Henderson94, Reference Shorvon, Carney and Chanarin95), and to a much lesser extent vitamin B6(Reference Carney, Ravindran and Rinsler96), have been linked with poorer psychiatric wellbeing. These B-vitamins play crucial roles in one-carbon metabolic pathways where they act as co-factors in DNA synthesis and repair, amino acid metabolism and methylation reactions, including the remethylation of homocysteine to methionine and subsequent generation of S-adenosylmethionine. S-adenosylmethionine, the universal methyl donor, is involved in the methylation of DNA, phospholipids, proteins and neurotransmitters, thus reduced status of one or more of the B-vitamins involved in one-carbon metabolism may impair methylation processes(Reference Selhub, Bagley and Miller97, Reference Bottiglieri, Laundy and Crellin98). The inhibition of methylation reactions may in turn influence cognitive impairment in ageing in various ways(Reference Smith and Refsum99), by perturbing the regulation of gene expression in the β-amyloid pathway, by reducing the activity of protein phosphatase-2A or by impairing the formation of phosphatidylcholine-enriched n-3 fatty acids(Reference Smith and Refsum99). Additionally, reduced tissue concentration of S-adenosylmethionine may be linked to depression through perturbing monoamine (serotonin, dopamine and noradrenaline) synthesis and methylation(Reference Bottiglieri, Laundy and Crellin98). Apart from folate, vitamins B12 and vitamin B6, which have well-recognised roles in these pathways, riboflavin (in its cofactor forms flavin adenine dinucleotide and FMN) is also essential in one-carbon metabolism but its potential role in influencing brain health has rarely been considered.
Numerous observational studies have shown that lower status of folate, vitamin B12 and vitamin B6 (and/or higher concentrations of homocysteine) are associated with cognitive deficit in ageing as extensively reviewed elsewhere(Reference Smith and Refsum99, Reference Porter, Hoey and Hughes100). Randomised trials in older adults that include intervention with high-dose folic acid, vitamin B12 and vitamin B6 over 2 years or more have shown, not only improved cognitive performance(Reference Durga, van Boxtel and Schouten101–Reference Douaud, Nichols and Smith104), but also a reduced rate of brain atrophy in studies which have incorporated MRI(Reference Smith, de Jager and Whitbread103, Reference Douaud, Nichols and Smith104). Notably the greatest slowing in atrophy (53 %) was seen among participants with MCI and the highest homocysteine concentrations at baseline (>13 µmol/l), while cognitive function was preserved in those supplemented with B-vitamins and with a baseline homocysteine concentration >11·3 µmol/l(Reference de Jager, Oulhaj and Jacoby102). The RCT evidence is not entirely consistent, however, as one recent and rather controversial meta-analysis in this area concluded that neither folic acid nor vitamin B12 had a beneficial effect on cognition in older adults(Reference Clarke, Bennett and Parish105). This paper was however widely criticised at the time of publication, mainly as a result of the inclusion criteria used to select the trials for investigation, and thus the findings are in general not widely accepted by experts in this area(Reference Garrard and Jacoby106, Reference Smith, de Jager and Refsum107). It is clear that further appropriately designed randomised trials are needed, especially those targeting participants with low B-vitamin status (and in those at most risk of cognitive decline) as they are likely to benefit the most from optimising B-vitamin concentrations to achieve better cognitive health in ageing. Furthermore, research investigating the protective role of riboflavin on cognitive function is greatly lacking, albeit some evidence from older studies investigating riboflavin showed that lower biomarker status was associated with cognitive impairment(Reference Xiu, Wahlqvist and Li108). Clearly there is a need for riboflavin to be considered in future randomised trials.
The role of B-vitamins in depressive disorders has not received as much interest as studies of cognitive disorders, although some observational (Table 1) and intervention (Table 2) evidence exists. A meta-analysis of nineteen observational studies concluded that low folate status was associated with a significantly greater risk of depression(Reference Gilbody, Lightfoot and Sheldon109). Low dietary intakes(Reference Sánchez-Villegas, Doreste and Schlatter110, Reference Skarupski, Tangney and Li111) or biomarker status(Reference Robinson, O'Luanaigh and Tehee112–Reference Reynolds115) of vitamin B12 have also been linked with an increased risk of developing depression. Only a limited number of studies have considered the role of vitamin B6, but available evidence suggests an inverse association between vitamin B6 biomarker status (plasma pyridoxal 5′-phosphate) and depression(Reference Skarupski, Tangney and Li111, Reference Merete, Falcon and Tucker116, Reference Hvas, Juul and Bech117). Far less evidence exists in relation to riboflavin, although one early study reported lower biomarker status of riboflavin in psychiatric inpatients(Reference Carney, Ravindran and Rinsler96). A number of randomised trials have considered the role of B-vitamin supplementation alone(Reference Walker, Mackinnon and Batterham118–Reference Ford, Flicker and Thomas121) or as an adjunct to anti-depressant medications(Reference Coppen and Bailey122, Reference Almeida, Ford and Hirani123). The results are somewhat conflicting, however, and no clear conclusions have emerged, partly because of major methodological differences among studies. Reviews of the available evidence in relation to depression have concluded that folate and vitamin B12 may have roles in the longer term management of this condition(Reference Taylor, Carney and Goodwin124, Reference Almeida, Ford and Flicker125).
Table 1. Summary of observational studies investigating the association of B-vitamin intake and status with depression in older adults
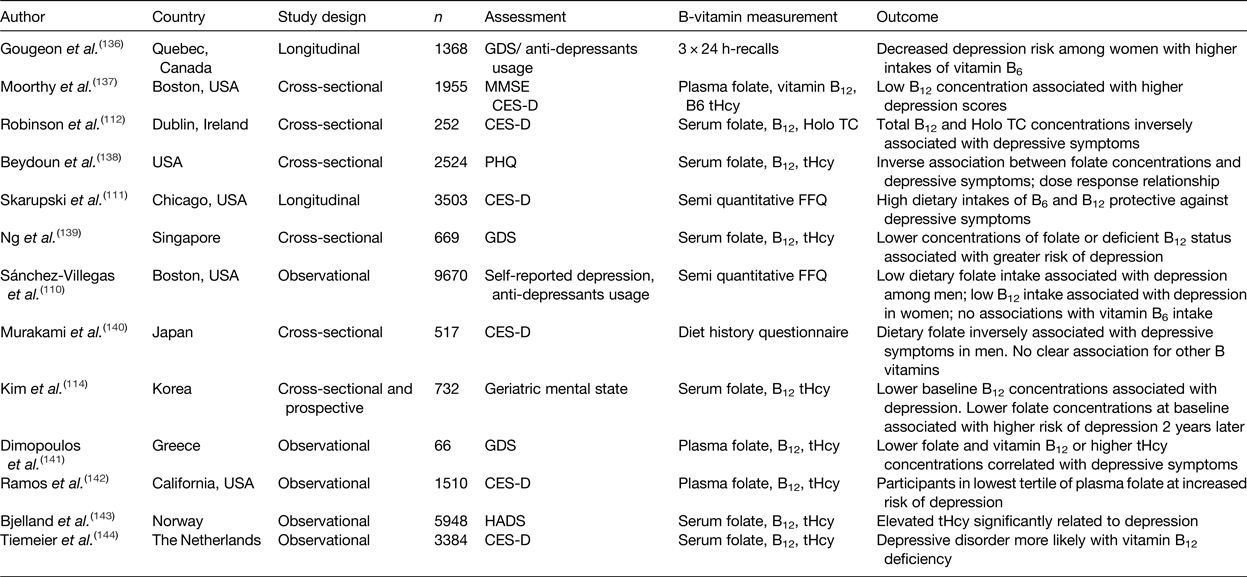
CES-D, centre for epidemiological studies depression scale; GDS, geriatric depression scale; HADS, hospital anxiety and depression scale; MMSE, mini mental state examination; PHQ, patient health questionnaire; Holo TC, holo-transcobalamin; tHcy, total plasma homocysteine.
Table 2. Summary of randomised controlled trials investigating the effect of B-vitamin supplementation on depression in older adults
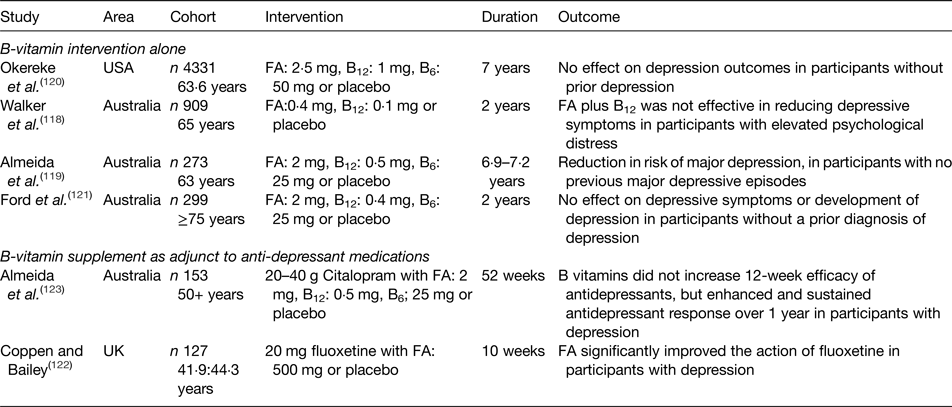
FA, folic acid.
Overall, there is considerable evidence to suggest that folate, vitamin B12 and vitamin B6 have protective effects on cognitive function, and potentially against depressive symptoms in ageing, however further randomised trials of appropriate duration in suitable populations, and ideally interventions combining all four relevant B-vitamins, are required to support these findings.
Use of novel imaging technologies in nutrition and brain research
Following the 2009 Nutrition and Mental Performance Task Force of the European Branch of the International Life Sciences Institute workshop, a recommendation was developed suggesting the inclusion of brain-imaging biomarkers as secondary endpoints to clinical and cognitive measures(Reference de Jager and Kovatcheva35). Brain-imaging techniques have been increasingly utilised in recent years and provide an objective and highly robust means of assessing brain structure, function and response to nutrition, with advantages and disadvantages associated with each of their use, as reviewed in detail elsewhere(Reference Sizonenko, Babiloni and de Bruin126) (Table 3). Electroencephalography and magnetoencephalography are two similar techniques for functional brain imaging and have the highest temporal resolution compared with other imaging techniques.
Table 3. Brain-imagining techniques for use in nutrition research
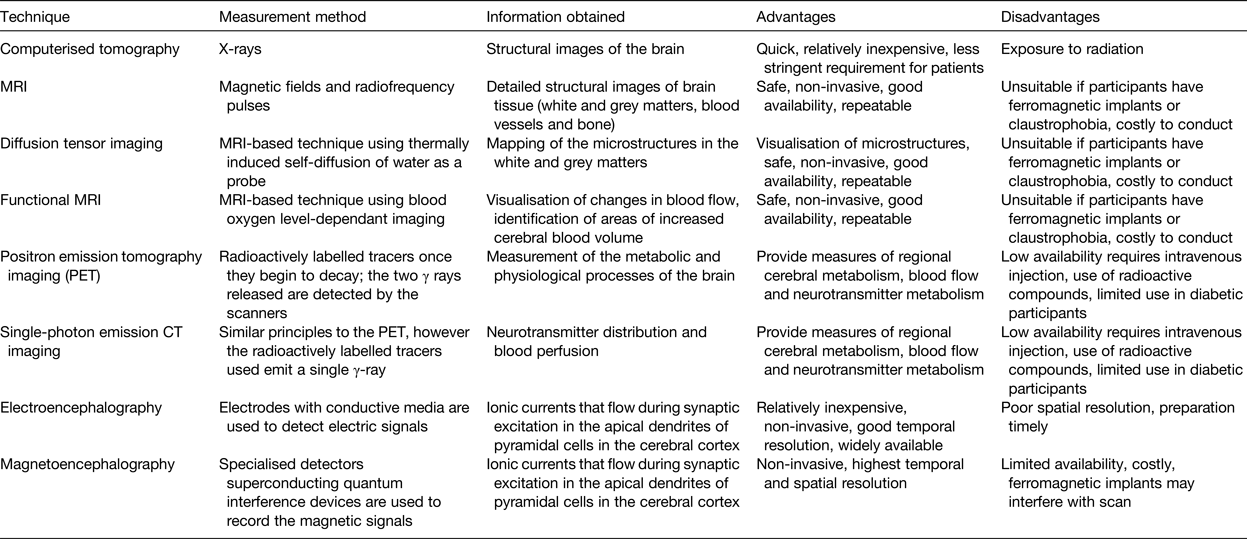
In recent years, some of these brain-imaging techniques have been utilised to advance nutrition research in ageing. One notable study referred to earlier in this review(Reference Smith, de Jager and Whitbread103) effectively used MRI and confirmed the beneficial effects of B-vitamins on cognition shown previously in older adults with MCI, in particular in those with good status of PUFA(Reference Jernerén, Elshorbagy and Oulhaj127). Additionally, Brickman et al. used functional MRI and demonstrated higher brain activation in specific regions of the brain in participants who consumed high-dose cocoa flavanols(Reference Brickman, Khan and Provenzano64). In a study of 239 older adults, diffusion tensor imaging (which in some cases has been suggested to be a better predictor of cognitive decline than other biomarkers)(Reference Selnes, Aarsland and Bjørnerud128), identified better white matter integrity in those who consumed more n-3 and n-6 PUFA and vitamin E(Reference Gu, Vorburger and Gazes129). Electroencephalography has also been used, with one recent report showing improved memory and functional connectivity in the δ band in response to Souvaid®, a nutritional supplement containing PUFA uridine, choline, phospholipids, folic acid, vitamin B6, B12, C, E and selenium in mild Alzheimer-type patients(Reference Ritchie, Bajwa and Coleman130). Positron emission tomography imaging has also been conducted within a 3-week intervention study, albeit in a very small study of only eleven women, leading to the conclusion that n-3 supplementation did not affect brain glucose metabolism in healthy older people(Reference Nugent, Pifferi and Fortier131).
It is clear that imaging techniques provide an objective means to improve the evidence base in this area, in particular in relation to proposed mechanisms. At this time, however, the number of studies utilising brain-imaging techniques to investigate the role of diet in brain health in ageing are limited. Magnetoencephalography has been approved by the US Food and Drug Administration for use within clinical and research settings as a means to assess and investigate cognitive dysfunction(Reference Maestú, Campo and Del Río132), AD(Reference de Haan, van der Flier and Koene133, Reference Cheng, Wang and Hsu134) and depression(Reference Kurita, Takei and Maki135). However, to our knowledge, no work has been published using magnetoencephalography for nutrition studies in older adults. The application of these new technologies in the field of nutrition, in combination with clinical and questionnaire-based assessments, could provide much potential for robust investigation in future studies, furthering knowledge and discovery.
Conclusions
Nutrition has important roles in preserving cognition and reducing the risk of late-life depression. Emerging evidence in this area implicates subclinical deficiencies of certain nutrients in cognitive decline and depression in older adults. Future studies should address the gaps in the literature, in particular in identifying of the threshold for optimal nutrient levels required to prevent or delay declining brain health. At this time, the evidence for potential protective effects is strongest in relation to B-vitamins, n-3 PUFA and polyphenols. If confirmed, a public health strategy to improve status of these key nutrients may help to achieve better cognitive and mental health and thus improve quality of life in older age. Future well-designed randomised trials (ideally incorporating imaging techniques such as magnetoencephalography) may provide a more robust basis for confirming effective nutrition interventions, which if implemented could reduce the risk of cognitive and mental health disorders in ageing and the related burden on health services and society overall.
Acknowledgements
The PhD studentship of K. M. was funded by the Northern Ireland Department for Employment and Learning who had no role in the design, analysis or writing of the present paper.
Financial Support
This work was supported by funding from the Northern Ireland Department for Employment and Learning, which funded the PhD studentship for K. M. The Northern Ireland Department for Employment and Learning had no role in the design, analysis or writing of the present paper.
Conflicts of Interest
None.
Authorship
K. M. drafted the manuscript. H. McN., C. F. H., L. H. and M. W. critically revised the manuscript for important intellectual content. All the authors have read and approved the final manuscript.








