Introduction
Taenia solium cysticercosis is a severe zoonotic disease, which causes serious public health problems and provokes economic losses in the areas where it is endemic, particularly in developing countries (Zoli et al., Reference Zoli, Shey-Njila, Assana, Nguekam, Dorny, Brandt and Geerts2003; Carabin et al., Reference Carabin, Krecek, Cowan, Michael, Foyaca-Sibat, Nash and Willingham2006; Praet et al., Reference Praet, Speybroeck, Manzanedo, Berkvens, Nsame Nforninwe, Zoli, Quet, Preux and Geerts2009). The important findings concerning the control of T. solium cysticercosis during the past two decades were reviewed by Pawlowski et al. (Reference Pawlowski, Allan and Sarti2005). One of the most important conclusions is that tapeworm carriers and human and porcine cysticercosis cases cluster in endemic foci. The risk factors for human cysticercosis are frequent consumption of pork, poor hygiene and having a history of taeniasis (Flisser & Gyorkos, Reference Flisser and Gyorkos2007). Although the presence of free-roaming pigs in rural areas of developing countries was identified as an important risk factor for swine cysticercosis (Sikasunge et al., Reference Sikasunge, Phiri, Phiri, Dorny, Siziya and Willingham2007), this is not the only way for pigs to get access to human faeces. Direct defecation of pig keepers in pig pens has been reported in the west of Cameroon (Zoli et al., Reference Zoli, Geerts, Vervoort, Geerts, Kumar and Brandt1987; Shey-Njila et al., Reference Shey-Njila, Zoli, Awah-Ndukum, Nguekam, Assana, Byambas, Dorny, Brandt and Geerts2003). The Mayo-Danay division is the most important region for pig breeding in northern Cameroon. In this part of the country, pigs are kept by non-Muslim people who interact with an important Muslim community. Pig husbandry is traditional and involves mainly local breeds which receive some domestic waste and little feed supplement (Njoya et al., Reference Njoya, Awa, Moussa, Ngo Tama, Cardinale, Ebangi, Ngangué, Seiny, Poulain and Faure1997; Koussou, Reference Koussou1999). Limited data from earlier studies in northern Cameroon suggested hyperendemicity of human and porcine cysticercosis (Awa et al., Reference Awa, Njoya, Ngo Tama and Ekue1999, Assana et al., Reference Assana, Zoli, Sadou, Nguekam, Voundou, Pouedet, Dorny, Brandt and Geerts2001), but details are not known concerning the factors that predispose to the infection of pigs. The main objective of this study was to update data on swine cysticercosis and identify risk factors for this zoonosis in the Mayo-Danay division.
Materials and methods
Study area
The Mayo-Danay division is located in the far northern province of Cameroon (fig. 1). The annual average rainfall varies from 600 to 800 mm and the wet season lasts 5 months – from May to October – while little or no rain falls during the rest of the year. Temperatures vary from maximum of around 45°C to a minimum of 20°C. The total population is about 600,000 inhabitants. There are four predominant ethnic groups: the Massa, the Toupouri, the Mousgoum and the Moussey, who are largely animistic or Christian and primarily involved in crop and livestock production. Livestock activities include keeping of cattle, sheep, goats, poultry and pigs. Pig raising is possible because most of the population is non-Muslim.

Fig. 1 Map showing the study area (Mayo-Danay division, grey shading) in the north of Cameroon (all other Cameroon districts shown as white areas).
Survey design and data collection
A survey was conducted in Mayo-Danay division between June 2007 and April 2008. A one-visit, multiple-objects survey was carried out to collect information on the pig-farming system. One hundred and fifty households, keeping 1756 pigs, were randomly sampled out of the registries kept by the authorities in the major area of pig farming. These rural areas were identified following the preliminary study carried out by Assana et al. (Reference Assana, Zoli, Sadou, Nguekam, Voundou, Pouedet, Dorny, Brandt and Geerts2001). Three hundred and ninety-eight (398) blood samples from pigs were collected in these surveyed households. A preliminary study had shown that the seroprevalence of pig cysticercosis in Mayo-Danay was 39.8% (Assana et al., Reference Assana, Zoli, Sadou, Nguekam, Voundou, Pouedet, Dorny, Brandt and Geerts2001). Assuming that a maximum sample size of 384 is required at the prevalence level of 50% (Sikasunge et al., Reference Sikasunge, Phiri, Phiri, Siziya, Dorny and Willingham2008), at least 368 pigs were needed in this study according to the formula n = Z 2PQ/L 2, where n is the required sample, Z is the Z score for confidence, P is the known prevalence, Q = (1 − P) and L is the error estimation at 95% confidence (Martin et al., Reference Martin, Meek and Willeberg1987). Therefore, a sample size of 398 pigs is easily sufficient to estimate the prevalence of pig cysticercosis in Mayo-Danay division.
Serology
Serum samples
Serum samples collected from pigs during the survey were stored at − 20°C until tested. Two positive serum samples from naturally infected pigs and eight negative serum samples from pigs from a local commercial pig farm without any history of cysticercosis were included as reference control sera in the enzyme-linked immunosorbent assay (ELISA).
Enzyme-linked immunosorbent assay for the detection of circulating T. solium antigens (Ag-ELISA)
The Ag-ELISA was performed as described by Brandt et al. (Reference Brandt, Geerts, De Deken, Kumar, Ceulemans, Brijs and Falla1992) and modified by Dorny et al. (Reference Dorny, Phiri, Vercruysse, Gabriel, Willingham, Brandt, Victor, Speybroeck and Berkvens2004). Briefly, the sera were pre-treated using trichloroacetic acid (TCA) and used in ELISA at a final dilution of 1/4. The monoclonal B158 was diluted at 5 μg/ml in carbonate buffer (0.06 m, pH 9.6) for coating the ELISA plate and the biotinylated monoclonal antibody (MoAb) B60 (1.25 μg/ml in phosphate-buffered saline containing 0.05% Tween 20 (PBS-T20)/1% newborn calf serum (NBCS)) was included as detector antibody. The incubation was carried out at 37°C on a shaker for 30 min, for the coating of the first MoAb, and for 15 min for all subsequent steps. The chromogen/substrate solution, consisting of orthophenylene diamine (Dako, Glostrup, Denmark) and H2O2 was added and incubated without shaking between 30 and 33°C for 15 min. To arrest the reaction, 50 μl of 4 N H2SO4 was added to each well. The plates were read using an ELISA reader (Multiskan RC, MTX Lab Systems, Vienna, Virginia, USA) at 492 nm.
Enzyme-linked immunosorbent assay for the detection of antibodies against T. solium cysticerci (Ab-ELISA)
The ELISA for the detection of antibodies against T. solium cysticerci (Ab-ELISA) was based on a 14 kDa antigen (F3 antigen) purified from T. solium cyst fluid by a two-step chromatography, as described by Assana et al. (Reference Assana, Kanobana, Tume, Zoli, Nguekam, Geerts, Berkvens and Dorny2007). The antigen (F3) was diluted at 1 μg/ml in a carbonate buffer (0.06 m, pH 9.6) for coating (1 h at 37°C and overnight at 4°C). PBS-T20 was used for washing between steps. Serum samples and conjugate were blocked and incubated on a shaking plate for 1 h at 37°C. PBS–T20/NBCS was used for blocking. Serum samples were diluted at 1/300. A rabbit anti-pig IgG peroxidase conjugate (Sigma, St. Louis, Missouri, USA) was used at a dilution of 1/30,000. The wells were washed three times, and the final steps were executed as described for the Ag-ELISA.
For both Ag- and Ab-ELISA, the optical density (OD) of each serum sample was compared with a series of reference negative serum samples (n = 8) at a probability level of P = 0.001 to determine the cut-off, using a modified Student's t-test (Sokal & Rohlf, Reference Sokal and Rohlf1981).
Statistical analysis
A Bayesian analysis was used to estimate the true prevalence of cysticercosis in the Mayo-Danay division and the sensitivity (Se) and specificity (Sp) of both Ag-ELISA and Ab-ELISA tests using a multinomial model as described by Krecek et al. (Reference Krecek, Michael, Schantz, Ntanjana, Smith, Dorny, Harrison, Grimm, Praet and Willingham2008). Briefly the results from Ag-ELISA and Ab-ELISA were combined together in a Bayesian model and run in Wingbugs 1.4 (Spiegelhalter et al., Reference Spiegelhalter, Thomas, Best and Lum2003). The prior information was obtained from previous studies on diagnosis of cysticercosis (Pouedet et al., Reference Pouedet, Zoli, Nguekam, Vondou, Assana, Speybroeck, Berkvens, Dorny, Brandt and Geerts2002; Dorny et al., Reference Dorny, Phiri, Vercruysse, Gabriel, Willingham, Brandt, Victor, Speybroeck and Berkvens2004; Assana et al., Reference Assana, Kanobana, Tume, Zoli, Nguekam, Geerts, Berkvens and Dorny2007). The sensitivity and specificity of the Ag-ELISA were constrained to intervals (0.8, 1) and (0.9, 1), respectively, whereas the sensitivity and specificity of the Ab-ELISA were constrained to intervals (0.4, 1) and (0.5, 1). The validation of the model was based on the number of parameters (P d), the Deviance Information Criterion (DIC) values from posterior mean of the multinomial probabilities (DIC_Pr) and from posterior mean of the parameter of the model using parent nodes (DIC_P) and on the Bayesian-p values (Bayesp) as described by Berkvens et al. (Reference Berkvens, Speybroeck, Praet, Adel and Lesaffre2006). To identify risk factors for pig cysticercosis, logistic regression was performed as described by Prasad et al. (Reference Prasad, Prasad, Gupta, Pandey and Singh2007). A pig that had a positive result in the Ag- or Ab-ELISA was considered as an infected pig in the analysis of risk factors.
Results
Pig-farming systems and sanitary facilities
Most of the pig farmers (90.7%) in the Mayo-Danay division kept pigs in a free-roaming system during the dry season. In the rainy season, pigs were confined in a small pig pen. From the 150 farmers interviewed, only 4.9% practised permanent indoor pig raising, whereas 13.7% allowed the pigs to roam freely during the rainy season (since there is sometimes overlapping of various systems, the total figure exceeds 100%). The survey showed that 16% of the households did not have a pig pen. Furthermore, it revealed that 42.7% of the households keeping pigs had no latrine facilities and that 76.0% of the pig farmers declared that the members of their family used open-field defecation.
Serology
The overall seroprevalence of pig cysticercosis was 24.6 and 32.2% for Ag-ELISA and Ab-ELISA, respectively. Of the total of 150 households surveyed, 56.0% and 57.3% of them had at least one pig positive for porcine cysticercosis in Ag-ELISA and Ab-ELISA, respectively.
Bayesian analysis
There was a good correlation between the results obtained by Ag-ELISA and Ab-ELISA. Two hundred and twenty-eight out of 398 sera were negative in both Ag- and Ab-ELISA, whereas 56 out of 398 sera were positive in both tests. The estimated true prevalence using Bayesian analysis was 26.6% (95% CI: 15.6–31.0%). Forty-two sera were only positive in the Ag-ELISA and 72 only in the Ab-ELISA. The sensitivity and specificity of the Ag-ELISA and Ab-ELISA used for the diagnosis of pig cysticercosis in the Mayo-Danay division are presented in table 1. The validation criteria of the Bayesian model are shown in table 2.
Table 1 The true prevalence of porcine cysticercosis in Mayo-Danay and the sensitivity (Se) and specificity (Sp) of Ag-ELISA and Ab-ELISA estimated by means of Bayesian analysis (with 95% confidence intervals).

Table 2 Model validation based on Bayesp, DIC and P d used to estimate the prevalence of cysticercosis in Mayo-Danay division and the sensitivity and specificity of Ag-ELISA and Ab-ELISA.
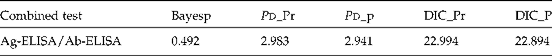
Bayesp, Bayesian-p values; DIC, Deviance Information Criterion; DIC_Pr, DIC values from posterior mean of the multinomial probabilities; DIC_P, DIC values from posterior mean of the parameter of the model using parent nodes.
Risk factor analysis
The factors that were considered as significant risks for pig cysticercosis are presented in table 3. The seroprevalence of cysticercosis in pigs was found to be associated with the pig owner's level of knowledge about the taeniasis–cysticercosis complex (odds ratio (OR) = 0.360, P < 0.05) and pig pen availability (OR = 0.45, P < 0.05).
Table 3 Univariate analysis of risk factors associated with seroprevalence of porcine cysticercosis in Mayo-Danay.

Discussion
Risk factors for porcine cysticercosis
In 1980, the pig population in Cameroon was estimated at more than 1,000,000 and 60% of the pigs were located in the western part of the country. After an outbreak of African swine fiver (ASF) in 1982 (Nana-Nukechap & Gibbs, Reference Nana-Nukechap and Gibbs1985), the pig population decreased in this part of the country. Since 1990, the north of Cameroon, free from ASF, emerged as the main provider of pork for the city of Yaoundé (Ndebi & Ongla, Reference Ndebi and Ongla2006). Pigs are kept in a traditional low-input breeding system (Njoya et al., Reference Njoya, Awa, Moussa, Ngo Tama, Cardinale, Ebangi, Ngangué, Seiny, Poulain and Faure1997), which favours infection with T. solium (Awa et al., Reference Awa, Njoya, Ngo Tama and Ekue1999). The results of this survey showed that 42.7% of households keeping pigs in the rural areas have no latrine facility and 76% of pig farmers use open-field defecation around the households. Even when a latrine was available this did not imply that it was used by the members of the household. In fact, these latrines were built to avoid problems with the sanitary authority, which controls the availability of latrines in the villages on a regular basis.
The coexistence of poor sanitary conditions and free roaming of domestic pigs (absence of pig pens; OR = 0.45; P < 0.05) certainly plays an important role in the circulation of T. solium infection in the region. Free roaming of pigs is known to be an important risk factor for infection of pigs with T. solium (Sikasunge et al., Reference Sikasunge, Phiri, Phiri, Dorny, Siziya and Willingham2007). This is not the case in the west and north-west of Cameroon where direct defecation by people in pig pens is an important factor associated with transmission of cysticercosis (Zoli et al., Reference Zoli, Geerts, Vervoort, Geerts, Kumar and Brandt1987; Shey-Njila et al., Reference Shey-Njila, Zoli, Awah-Ndukum, Nguekam, Assana, Byambas, Dorny, Brandt and Geerts2003). In addition, pigs are privately slaughtered without meat inspection. When answering the questionnaire, pig keepers knew and recognized pig cysticercosis through tongue examination or in pig meat, but did not know the relationship between taeniasis in humans and the presence of cysticerci in pigs. This lack of knowledge about the transmission of T. solium (OR = 0.360; P < 0.05) may contribute to the maintenance of a high prevalence of pig cysticercosis in the region.
Prevalence of porcine cysticercosis
In an earlier epidemiological survey conducted in the Mayo-Danay division in 1999, 38.9% of the pigs were found to be seropositive using an ELISA for antigen detection (Assana et al., Reference Assana, Zoli, Sadou, Nguekam, Voundou, Pouedet, Dorny, Brandt and Geerts2001). The results of the ELISAs in the present study (24.6% seropositive by Ag-ELISA and 32.2% by Ab-ELISA) are somewhat lower but confirm that pigs are still highly exposed to T. solium cysticercosis. However, neither of the tests used for the diagnosis of cysticercosis is perfect (Dorny et al., Reference Dorny, Phiri, Vercruysse, Gabriel, Willingham, Brandt, Victor, Speybroeck and Berkvens2004, Krecek et al., Reference Krecek, Michael, Schantz, Ntanjana, Smith, Dorny, Harrison, Grimm, Praet and Willingham2008). The Ab-ELISA, using a 14 kDa purified antigen (F3), is more specific but less sensitive than the Ag-ELISA (Assana et al., Reference Assana, Kanobana, Tume, Zoli, Nguekam, Geerts, Berkvens and Dorny2007). It measures the exposure of the pigs to T. solium, but this does not imply that cysticerci are present. The Ag-ELISA, on the other hand, detects the presence of living cysts, but cross-reacts with Taenia hydatigena. The advantage of using both the Ab-ELISA and the Ag-ELISA and analysing the results in a Bayesian model is that the F3 antigen used in the former test does not react with serum samples from pigs infected with T. hydatigena (Assana et al., Reference Assana, Kanobana, Tume, Zoli, Nguekam, Geerts, Berkvens and Dorny2007). However, the occurrence of T. hydatigena is not well known in northern Cameroon and should be studied further. The seroprevalence of cysticercosis using Ab-ELISA was higher than when using Ag-ELISA. This seems to indicate that a proportion of the pigs harbour only dead cysts or have specific transient antibodies against cysticercosis (Garcia et al., Reference Garcia, Gonzalez, Gilman, Palacios, Jimenez, Rodrigues, Verastegui, Wilkins and Tsang2001). In our study, 37% (42/114) of the serum samples positive in Ag-ELISA tested negative in Ab-ELISA. This discrepancy might be due to the lower sensitivity of the Ab-ELISA or to false-positive results in Ag-ELISA due to cross-reaction with T. hydatigena. Similar high proportions of positive sera in antigen detection found negative in antibody detection were obtained with Ab-ELISA using crude antigen from T. solium (Pouedet et al., Reference Pouedet, Zoli, Nguekam, Vondou, Assana, Speybroeck, Berkvens, Dorny, Brandt and Geerts2002), crude antigen from T. crassiceps (Dorny et al., Reference Dorny, Phiri, Vercruysse, Gabriel, Willingham, Brandt, Victor, Speybroeck and Berkvens2004) and also in a recent study using an enzyme-linked immunoelectrotransfer blot assay (EITB) based on affinity purified glycoproteins from T. solium cysticerci (Krecek et al., Reference Krecek, Michael, Schantz, Ntanjana, Smith, Dorny, Harrison, Grimm, Praet and Willingham2008). In the absence of data from autopsy (slicing of all muscles), which is the gold standard, it is possible to use conditional dependence between two or more imperfect tests to estimate the true prevalence of a disease and its test parameters by using a Bayesian analysis (Dendukuri & Joseph, Reference Dendukuri and Joseph2001; Berkvens et al., Reference Berkvens, Speybroeck, Praet, Adel and Lesaffre2006). In this study, we used conditional dependence between Ag-ELISA and Ab-ELISA to estimate the true prevalence of cysticercosis in the Mayo-Danay division. The Bayesian analysis indicated a true prevalence of 26.6%. This result is higher than the figure of 12% found by Pouedet et al. (Reference Pouedet, Zoli, Nguekam, Vondou, Assana, Speybroeck, Berkvens, Dorny, Brandt and Geerts2002) in western Cameroon, using a similar approach. The Bayesian analysis of these data revealed the sensitivity and specificity of the Ag-ELISA to be higher than those found with the Ab-ELISA (table 1). However, the diagnostic characteristics of the Ab-ELISA using F3 antigen and the EITB, the assay most commonly used for antibody detection in the diagnosis of cysticercosis (Tsang et al., Reference Tsang, Brand and Boyer1989), seem to be similar to those reported by Krecek et al. (Reference Krecek, Michael, Schantz, Ntanjana, Smith, Dorny, Harrison, Grimm, Praet and Willingham2008), who used the same Bayesian approach.
In conclusion, this study clearly shows that porcine cysticercosis is hyperendemic in Mayo-Danay and that the pig-farming system contributes to maintaining this high level of infection. This situation calls for preventive measures to control pig cysticercosis in the region.
Acknowledgements
This study was supported by the Wellcome Trust (United Kingdom) through project 075818; from the National Health and Medical Research Council (Australia) grant 350279; and, in part, by pig project funds from the Diocese of Yagoua (Cameroon) and the Ministry of Livestock, Fisheries and Animal industries (Cameroon). We acknowledge the help of R. De Deken in preparing the figure.








