Coffee is among the most widely consumed beverages in the world. The relationship between coffee drinking and CVD has been extensively studied. Recently several studies indicate a moderate coffee consumption can be protective against cardiovascular risk(Reference Kleemola, Jousilahti, Pietinen, Vartiainen and Tuomilehto1–Reference Andersen, Jacobs, Carlsen and Blomhoff3). The effect of coffee on platelet aggregation has been poorly studied. Although the main physiologic effects resulting from coffee consumption are usually ascribed to the presence of caffeine, coffee is also an extremely rich source of phenolic acids, mostly in the form of chlorogenic acid. The chlorogenic acid content of coffee is very similar to that of caffeine; a cup of American coffee contains, in fact, about 170 mg chlorogenic acid and 180 mg caffeine(Reference Natella, Nardini, Giannetti, Dattilo and Scaccini4). The daily intake of chlorogenic acid in coffee drinkers ranges from 0·5 to 1·0 g(Reference Clifford, Clarke and Macrae5), and the presence of coffee phenolic acids in plasma after coffee drinking is well documented(Reference Nardini, Cirillo, Natella and Scaccini6).
Several epidemiological studies have reported a negative association between the intake of dietary polyphenols and the risk for CVD(Reference Hertog, Kromhout, Aravanis, Blackburn, Buzina, Fidanza, Giampaoli, Jansen, Menotti and Nedeljkovic7, Reference Arts, Hollman, Feskens, Bueno de Mesquita and Kromhout8). The activity of dietary polyphenols on CVD has been attributed to both their antioxidant activity in preventing LDL oxidation, and to their effect on the pathogenesis of thrombosis by interfering with platelet activation and function. Platelets play an important role not only in the formation of acute thrombus and vessels occlusion, but also in the development of atherosclerosis and CVD(Reference Fuster, Badimon, Badimon and Chesebro9). Moreover, ex vivo platelet aggregation is related to CVD mortality(Reference Thaulow, Erikssen, Sandvik, Stormorken and Cohn10).
Phenolic compounds are capable of inhibiting platelet aggregation both in vitro (Reference Freedman, Parker, Perlman, Frei, Ivanov, Deak, Iafrati and Folts11, Reference Olas, Wachowicz, Saluk-Juszczak and Zielinski12) and in vivo (Reference Demrow, Slane and Folts13, Reference Luceri, Giannini, Lodovici, Antonucci, Abbate, Masini and Dolara14). It has been demonstrated that phenolic-rich beverages, such as cocoa, tea and wine, can inhibit platelet aggregation both in vitro (Reference Freedman, Parker, Perlman, Frei, Ivanov, Deak, Iafrati and Folts11, Reference Pignatelli, Pulcinelli, Celestini, Lenti, Ghiselli, Gazzaniga and Violi15, Reference Lill, Voit, Schror and Weber16) and ex vivo (Reference Freedman, Parker, Perlman, Frei, Ivanov, Deak, Iafrati and Folts11, Reference Hodgson, Puddey, Burke, Beilin, Mori and Chan17, Reference Pignatelli, Lenti, Pulcinelli, Catasca, Saccani, Germano, Marcoccia, Silvestri, Ghiselli and Violi18).
In 1987 Bydlowski et al. (Reference Bydlowski, Yunker, Rymaszewski and Subbiah19) demonstrated that coffee extracts could inhibit in vitro platelet aggregation induced by ADP or arachidonic acid, but not by collagen, and were effective in inhibiting platelet aggregation ex vivo after intravenous administration in rabbits. More recently, Polagruto et al. (Reference Polagruto, Schramm, Wang-Polagruto, Lee and Keen20) did not find any effect on platelet function (assessed by a Platelet Function Analyser PFA-100) in man 2 or 6 h after instant coffee consumption.
The aim of the present study was to: (1) evaluate ex vivo the effect of coffee on platelet aggregation and (2) investigate in vivo the platelet uptake of coffee phenolic acids. As both caffeine(Reference Rein, Paglieroni, Wun, Pearson, Schmitz, Gosselin and Keen21, Reference Varani, Portaluppi, Gessi, Merighi, Ongini, Belardinelli and Borea22) and phenolics(Reference Freedman, Parker, Perlman, Frei, Ivanov, Deak, Iafrati and Folts11, Reference Violi, Pignatelli and Pulcinelli23) have been suggested to affect platelet aggregation, the present study was performed using caffeine as control.
Subjects and methods
Subjects and study design
Ten volunteers (five males and five females), aged 24–35 years, participated in a cross-over study. Subjects, free from known diseases and moderate coffee drinkers (two to four cups/d), were instructed to refrain from consuming coffee and phenol-rich foods and beverages for the 2 d prior to the experiments. Subjects were also asked to abstain from any platelet inhibitor and dietary supplements for ≥ 10 d prior to the experiments. Subjects reported to the laboratory on two separate occasions, 2–4 weeks apart, after an overnight fast (10–12 h). A venous blood sample was taken at time 0. Immediately after the first blood collection subjects were provided with a cup of freshly prepared American coffee (200 ml) or a capsule containing 180 mg caffeine with 200 ml water. This dose was equivalent to the caffeine contained in the cup of coffee. Further blood collections were made 30 and 60 min after coffee or caffeine consumption. Venous blood samples were collected into evacuated tubes containing sodium citrate. Platelet-rich plasma (PRP) was separated by low-speed centrifugation (180 g, 15 min) at room temperature.
All subjects gave informed consent before entering the study, and all procedures were approved by the Ethical Committee of the National Institute for Food and Nutrition Research.
Coffee brew preparation and analyses
Coffee brew was prepared by using a commercial automatic brewing machine, using 60 g roasted and ground coffee per litre of water.
Caffeine was measured in coffee by HPLC as previously described by Blanchard et al. (Reference Blanchard, Mohammadi and Conrad24), and the amount found after three replicate analyses (180 (sem 9) mg/cup) was used for caffeine capsule formulation.
Measurement of plasma caffeine concentrations
Caffeine was detected in plasma and platelets by HPLC as previously described(Reference Blanchard, Mohammadi and Conrad24).
Measurement of platelet aggregation
Platelet aggregation was measured ex vivo on PRP using an aggregometer, with constant stirring at 1000 rpm and at 37°C. PRP was separated from blood after 20 min centrifugation at 200 g and the number of platelets in the PRP was standardized at 300 000 platelets/ml. To induce platelet aggregation, three different agonists were used: ADP (2 μm), collagen (3 μg/ml) and arachidonic acid (0·5 μm). Platelet aggregation was expressed as percentage of maximal aggregation.
For the in vitro experiments, we used PRP obtained from healthy subjects in fasting condition. PRP was standardized at 300 000 platelets/ml and pre-incubated (30 min at 37°C) with a mix of caffeic acid (2·4 ng/mg protein), ferulic acid (1·9 ng/mg protein), p-coumaric acid (1·8 ng/mg protein) and isoferulic acid (0·9 ng/mg protein), mimicking the concentrations of the phenolic acids measured in vivo. Platelet aggregation was induced by collagen (3 μg/ml) and measured as described earlier.
Measurement of platelet thromboxane B2 formation
Thromboxane B2 (TxB2) production was measured in platelets separated at time 0, 30 and 60 min after coffee drinking, on a sub-group of six subjects. PRP was incubated with collagen (3 μg/ml). The platelet activation was stopped after 10 min and thromboxane A2 (TxA2) production was determined using TxB2 (the stable breakdown product of TxA2) ELISA kits (R&D System).
Measurement of platelet phenolic acid concentrations
Phenolic acids in biological samples are routinely detected in our laboratory by HPLC-electrochemical detector(Reference Nardini, Natella, Scaccini and Ghiselli25). For the study of incorporation of phenolics in platelets, PRP was added with acid citrate dextrose and further centrifuged for 7 min at 780 g. The pellet was washed once with PBS containing acid citrate dextrose, centrifuged and the final pellet was resuspended in water, sonicated, acidified to pH 3 with 1 m-HCl and stored at − 80°C. The presence of phenolic acid into platelets was measured in samples untreated (free phenolic acids) and in samples subjected to alkaline hydrolysis (total phenolic acids)(Reference Nardini, Cirillo, Natella and Scaccini6).
No treatment
Platelet suspension (0·5 ml) at pH 3 was added with 50 ng o-coumaric acid as internal standard and 300 mg NaCl, then extracted three times with ethyl acetate ( × 4 volumes) by vortexing for 5 min. After each extraction, samples were centrifuged (3000 g, 10 min, room temperature) and the supernatants collected. The organic phase was dried under nitrogen flow. The residue was dissolved in 0·5 ml water, vortexed for 5 min, then the pH was brought to pH 7–8 with 0·1 m-NaOH and sample passed through 1 ml Supelclean LC-SAX tube preconditioned with 1 ml absolute methanol and 2 ml water. The tube was then washed with 1 ml water. Phenolic acids elution was obtained with 1 ml buffer containing 1 m-acetic acid–methanol (90:10). The eluant was immediately brought to pH 3 with 6 μl 4 m-NaOH, filtered and an aliquot (100 μl) was injected into the HPLC system.
Alkaline hydrolysis treatment
Platelet suspension (0·5 ml) containing 50 ng o-coumaric acid as internal standard had the following added in order: 55 μl H2O, 20 μl 0·5 m-EDTA, 200 μl 5 % ascorbic acid and 225 μl 8 m-NaOH, and was then incubated for 30 min at 30°C. At the end of incubation, the pH was brought to 3 with 8 m-HCl and 600 mg NaCl added. Samples were extracted three times with ethyl acetate ( × 4 volumes) as reported earlier. The residue was dissolved in 0·5 ml water, vortexed for 5 min, then processed for solid-phase extraction as described earlier.
The overall procedures allow an almost complete recovery of the phenolic acids under study, as found by recovery experiments performed adding known amounts of pure compounds to platelet suspension samples. Recovery was 98·6 (sd 9·9), 95·4 (sd 13·6), 87·6 (sd 4·4), 100·7 (sd 5·7) (n 5) for caffeic, p-coumaric, ferulic and isoferulic acids, respectively.
Samples were analysed by HPLC-electrochemical detector as previously described(Reference Nardini, Natella, Scaccini and Ghiselli25) with minor modifications concerning the elution gradient.
Protein was measured by the method of Lowry et al. (Reference Lowry, Rosebrough, Farr and Randall26), using bovine serum albumin as standard. The concentration of phenolic acids is expressed as ng/mg protein.
Free forms of phenolic acids in platelets were detected only at very low amounts (traces), therefore all the results presented refer to the total (free+bound) phenolic acid content.
Statistics
All data are presented as mean values with their standard errors. Statistical analysis was carried out using repeated-measures ANOVA, followed by Tukey's test for multiple comparisons. Analyses were performed with KaleidaGraph software version 3.6 (Synergy Software, Reading, PA, USA). P < 0·05 was considered statistically significant.
Results
Effect of coffee and caffeine consumption on ex vivo platelet aggregation
Coffee drinking significantly inhibited platelet aggregation induced by arachidonic acid and collagen (Fig. 1), while no statistically significant differences were observed when ADP was used as agonist (Fig. 1).
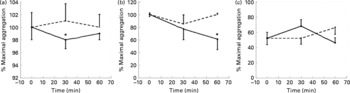
Fig. 1 Maximal platelet aggregation (%) at baseline, 30 and 60 min after coffee (–●–) and caffeine (- - -) consumption (n 10). The aggregation was induced by collagen (3 μg/ml) (a); arachidonic acid (0·5 μm) (b); ADP (2 μm) (c). Values are means with their standard errors depicted by vertical bars. Mean values were significantly different from those of time 0 (repeated-measures ANOVA followed by Tukey's test): *P < 0·05.
Coffee drinking also inhibited collagen-induced TxB2 formation (Fig. 2).
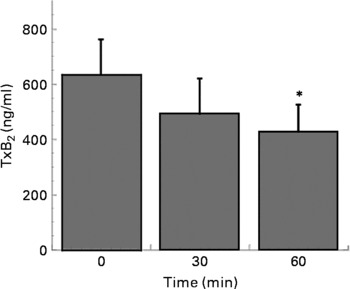
Fig. 2 Thromboxane B2 (TxB2) production in platelets separated at time 0, 30 and 60 min after coffee consumption (n 6). Platelets were activated using collagen (3 μg/ml). Values are means with their standard errors depicted by vertical bars. Mean values were significantly different from those of time 0 (repeated-measures ANOVA followed by Tukey's test): *P < 0·05.
Caffeine intake did not affect platelet aggregation induced by the three different agonists (Fig. 1).
Effect of coffee and caffeine supplementation on plasma caffeine concentrations
As expected, a statistically significant increase in plasma caffeine concentrations was detectable after both coffee and caffeine consumption (Fig. 3). However, the time course of caffeine increase was different in the two sessions, being slower after caffeine administration than after coffee consumption. Caffeine plasma concentrations measured after 30 min of caffeine intake (15·2 (sem 7·8) μm) were, in fact, statistically significantly different from those observed 30 min after coffee drinking (20·6 (sem 8·1) μm).
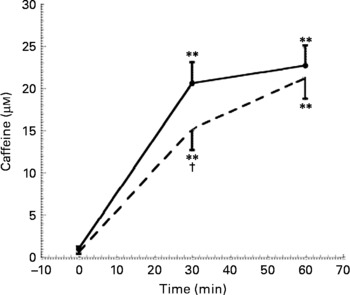
Fig. 3 Caffeine concentration at baseline, 30 and 60 min after coffee (–●–) and caffeine (- - -) consumption (n 10). Values are means with their standard errors depicted by vertical bars. Mean values were significantly different from those of time 0 (repeated-measures ANOVA followed by Tukey's test): **P < 0·01. Mean values were significantly different from those of coffee (repeated-measures ANOVA followed by Tukey's test): †P < 0·05.
Caffeine platelet concentration was under the detection limits of our method (5 ng).
Effect of coffee consumption on platelet phenolic acid concentration
Coffee drinking induced a significant increase in phenolic acid platelet concentration (Table 1). All phenolic acids under study increased significantly, but they showed different incorporation kinetics. In fact, the maximum incorporation peak was at 30 min for p-coumaric and ferulic acids, and at 60 min for caffeic and isoferulic acids. The maximum concentration reached after coffee drinking was: 2·4 ng/mg protein for caffeic acid, 1·9 ng/mg protein for ferulic acid, 1·8 ng/mg protein for p-coumaric acid and 0·9 ng/mg protein for isoferulic acid.
Table 1 Concentration of total phenolic acids (ng/mg protein) in platelets before and after coffee drinking (n 10) (Mean values with their standard errors)
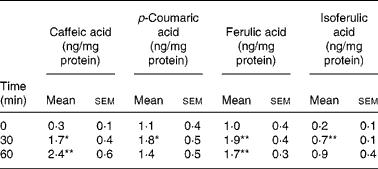
Mean values were significantly different from those of time 0 (repeated-measures ANOVA followed by Tukey's test): *P ≤ 0·05, **P ≤ 0·01.
The most relevant increases were observed for caffeic acid (8-fold at peak time with respect to time 0) and isoferulic acid (4·5-fold at peak time with respect to time 0). p-Coumaric and ferulic acids increased more slightly, the increment of their concentration at peak time being about 60 and 90 %, respectively.
Phenolic acids were present in platelets almost exclusively as conjugated forms, free phenolic acids being present only as traces.
Effect of phenolic acids on in vitro platelet aggregation
To evaluate the capacity of phenolic acids in inhibiting platelet aggregation, a mix of phenolic acids was added to PRP at the concentrations measured in platelets after coffee drinking. After 30 min of incubation at 37°C aggregation was induced by collagen (3 μg/ml). As shown in Fig. 4, the mix of phenolic acids was able to significantly inhibit platelet aggregation and collagen-induced TxB2 formation.
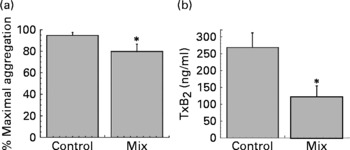
Fig. 4 Maximal platelet aggregation (%) (a) and thromboxane B2 (TxB2) release (b) (n 5). Platelet-rich plasma was pre-incubated for 30 min at 37°C alone (control) or with a mix of caffeic acid (2·4 ng/mg protein), ferulic acid (1·9 ng/mg protein), p-coumaric acid (1·8 ng/mg protein) and isoferulic acid (0·9 ng/mg protein). The aggregation was induced by collagen (3 μg/ml). Values are means with their standard errors depicted by vertical bars. Mean values were significantly different from those of the control (repeated-measures ANOVA followed by Tukey's test): *P < 0·05.
Discussion
The first aim of the present study was to determine the effects of coffee on ex vivo platelet aggregation induced by three different agonists (collagen, arachidonic acid and ADP), using caffeine as control. While coffee drinking inhibited collagen and arachidonic acid-induced platelet aggregation, caffeine intake did not affect platelet aggregation induced by any of the agonists (Fig. 1). Coffee and caffeine intake induced a similar increase in plasma caffeine concentrations, which reached the same peak concentration 60 min after both supplementations (Fig. 3). Even if the kinetic of absorption was different, we can exclude some effect of caffeine on platelet aggregation. In fact, 60 min after caffeine intake (when plasma caffeine concentration was equal to the concentration reached after coffee drinking), no effect of caffeine on platelet aggregation was observed. Similar results were obtained by Rein et al. (Reference Rein, Paglieroni, Wun, Pearson, Schmitz, Gosselin and Keen21), who did not find any effect on ADP-induced platelet aggregation after acute intake of caffeine (17 mg). On the contrary, Varani et al. (Reference Varani, Portaluppi, Gessi, Merighi, Ongini, Belardinelli and Borea22) observed that chronic caffeine intake could affect ADP-induced platelet aggregation, but only when the intake lasted longer than 1 week and was higher than 400 mg/d. According to the present results, Varani et al. (Reference Varani, Portaluppi, Gessi, Merighi, Ongini, Belardinelli and Borea22) did not observe any effect of caffeine on platelet aggregation after acute intake.
The capacity of coffee to inhibit platelet aggregation has been already demonstrated, but only in an animal model. Bydlowski et al. (Reference Bydlowski, Yunker, Rymaszewski and Subbiah19) demonstrated that an intravenous administration of coffee extracts inhibited ex vivo arachidonic acid and ADP-induced platelet aggregation in rabbits. In man, Polagruto et al. (Reference Polagruto, Schramm, Wang-Polagruto, Lee and Keen20) did not find significant modification of platelet function 2 and 6 h after coffee drinking. However, their results are scarcely comparable with the present data, as platelet function was estimated using a completely different methodology (the measure of the adrenalin/collagen or ADP/collagen induced clotting time) and time-points.
Apart from coffee, several papers report an effect of specific food items on platelet aggregation in vivo in man, both in acute and chronic studies. An antiplatelet effect has been demonstrated for fruit juice(Reference Polagruto, Schramm, Wang-Polagruto, Lee and Keen20), red wine(Reference Pignatelli, Lenti, Pulcinelli, Catasca, Saccani, Germano, Marcoccia, Silvestri, Ghiselli and Violi18), onion(Reference Hubbard, Wolffram, de Vos, Bovy, Gibbins and Lovegrove27), tea(Reference Hodgson, Puddey, Burke, Beilin, Mori and Chan17, Reference Steptoe, Gibson, Vuononvirta, Hamer, Wardle, Rycroft, Martin and Erusalimsky28) and tomato(Reference O'Kennedy, Crosbie, Whelan, Luther, Horgan, Broom, Webb and Duttaroy29) (for a review, see Nardini et al. (Reference Nardini, Natella and Scaccini30)). These findings support the role of the diet (as a whole and/or its single components) in the modulation of the platelet network and confirm the important role of diet in the prevention of CVD.
Looking for the molecular mechanism through which coffee can affect platelet aggregation, we measured the capacity of coffee drinking to affect collagen-induced platelet TxA2 formation. The present results demonstrated that coffee inhibited both collagen and arachidonic acid-induced platelet aggregation, but it had no effect on ADP-induced platelet aggregation. Differently from ADP, the platelet activation pathways induced by arachidonic acid and collagen are both mediated by cyclo-oxygenase, through the formation of TxA2, which further stimulates aggregation. As shown in Fig. 2, platelets collected 60 min after coffee drinking and activated with collagen released significantly less TxB2 than those collected before drinking. The present result is in agreement with literature data reporting that coffee inhibits in vitro TxB2 generation in rabbit platelets(Reference Bydlowski, Yunker, Rymaszewski and Subbiah19). Thus, coffee affects the signal transduction pathway, which leads to aggregation through the inhibition of one of the enzyme activities that lies upstream of the formation of TxB2 in the signalling cascade.
It is well known that phenolic compounds can inhibit in vitro and ex vivo platelet aggregation(Reference Freedman, Parker, Perlman, Frei, Ivanov, Deak, Iafrati and Folts11, Reference Violi, Pignatelli and Pulcinelli23). Their anti-aggregating activity has been attributed to several mechanisms, among them the capacity to inhibit TxA2 generation(Reference Tzeng, Ko, Ko and Teng31, Reference Guglielmone, Agnese, Nunez Montoya and Cabrera32). Coffee contains several phenolic compounds, which are absorbed and rapidly metabolized in man(Reference Nardini, Cirillo, Natella and Scaccini6, Reference Olthof, Hollman and Katan33). In a previous study we demonstrated that coffee drinking induces a significant increase in plasma caffeic acid, which reaches micromolar concentrations 1 h after coffee intake(Reference Nardini, Cirillo, Natella and Scaccini6).
The incorporation of polyphenols into circulating cells after the intake of a polyphenol-rich food has not been demonstrated yet. In the present study, we demonstrate for the first time that metabolites of phenolic acids are incorporated in human platelets after a single oral dose of coffee.
It is important to underline that the evaluation of the actual bioavailability of polyphenols and their subsequent interaction with cells and tissues is a prerequisite for the real understanding of their physiological effect. In fact, many of the effects claimed for these compounds are exerted inside the cells(Reference Oak, El Bedoui, Anglard and Schini-Kerth34, Reference Dasgupta and Milbrandt35), but, unfortunately, no direct evidence exists about the ‘physiological’ presence of these compounds in cells in in vivo studies. Information on polyphenol tissue distribution is still very scarce, and available data concern only animal models. Animal studies, mostly using radiolabelled compounds, have shown that phenolic compounds are able to penetrate tissues, particularly those in which they are metabolized (intestine and liver(Reference Suganuma, Okabe, Oniyama, Tada, Ito and Fujiki36–Reference de Boer, Dihal, van der Woude, Arts, Wolffram, Alink, Rietjens, Keijer and Hollman38); for a review, see Manach et al. (Reference Manach, Scalbert, Morand, Remesy and Jimenez39)). In tissues phenolic compounds occur prevalently in conjugated forms, indicating that the ingested phenolics are extensively and rapidly metabolized(Reference Abd El Mohsen, Kuhnle, Rechner, Schroeter, Rose, Jenner and Rice-Evans40, Reference Graf, Mullen, Caldwell, Hartley, Duthie, Lean, Crozier and Edwards41). To our knowledge, only three studies report data on the occurrence of polyphenols in human cells in vivo (Reference Hong, Kim, Kwon, Lee and Chung42–Reference Meyer, Bolarinwa, Wolfram and Linseisen44), and, among these, only one study evaluates their presence in human blood cells. In the present study, apigenin metabolites were found in human erythrocytes, but no increase in their cellular concentration was observed after apigenin-rich parsley consumption(Reference Meyer, Bolarinwa, Wolfram and Linseisen44).
With the exception of isoferulic acid, all the phenolic acids measured in platelets after coffee drinking were present in coffee brew, even if in bound forms as esters of quinic acid (data not shown). In coffee, the most abundant of these esters is chlorogenic acid, whose phenolic moiety is represented by caffeic acid(Reference Clifford, Clarke and Macrae5). Isoferulic acid is a metabolite of caffeic acid and is not present, as such, in coffee. Its increase after coffee drinking indicates that caffeic acid is extensively metabolized, but we cannot speculate if its metabolism takes place before or after the incorporation into platelets. All the phenolic acids measured into platelets were present in conjugated forms, indicating a wide and rapid metabolic process. Yet, preliminary evidence obtained with an enzymatic hydrolytic procedure seems to indicate that most of the phenolic acids are present in platelets as glucuronates and sulphates (data not shown).
It must be evidenced that phenolic acid concentration inside platelets is sufficiently high to explain coffee anti-aggregative effects. The sum of platelet phenolic acid concentration, in fact, reaches about 6 ng/mg protein at 30 and 60 min. A raw calculation (based on a mean platelet volume and protein content) lets us estimate that caffeic acid alone can reach an intracellular concentration of about 2 μm. To test if phenolic acids, at the concentration observed in platelets after coffee drinking, were able to affect platelet aggregation, we ran in vitro experiments, using a mix of the different phenolic acids at the concentrations measured in vivo. As shown in Fig. 4, the mix is capable of significantly inhibiting collagen-induced platelet aggregation and collagen-induced TxB2 formation. It is well known that phenolic acids are efficient antioxidants(Reference Nardini, D'Aquino, Tomassi, Gentili, Di Felice and Scaccini45) and caffeic acid is a good inhibitor of lipoxygenase(Reference de la Puerta, Ruiz Gutierrez and Hoult46), cyclo-oxygenase(Reference Huang, Lysz, Ferraro, Abidi, Laskin and Conney47) and kinases(Reference Nardini, Scaccini, Packer and Virgili48). All these activities could be critical for the anti-aggregative capacity of phenolic acids.
We are aware that phenolic acids incorporated into platelets are not in their free form, but in conjugated forms, likely glucuronates and sulphates. Since at the moment the appropriate standard compounds are not commercially available, we cannot determinate if these forms are more or less active than their respective free forms. Scarce information is available concerning the potential activity of the metabolites of polyphenols. Conjugated forms of quercetin, one of the most abundant flavonoids in the human diet, retain antioxidant activity, although to a lesser extent in respect to quercetin(Reference Manach, Morand, Crespy, Demigne, Texier, Regerat and Remesy49). The antioxidant activity of ferulic acid glucuronide is stronger than that exhibited by ferulic acid(Reference Ohta, Nakano, Egashira and Sanada50). However, some polyphenol metabolites (quercetin tetrasulphate and genistein sulphates) have been reported to have lower antiplatelet activities than their parent molecules(Reference Guglielmone, Agnese, Nunez Montoya and Cabrera32, Reference Rimbach, Weinberg and de Pascual-Teresa51). The possibility exists that the free forms penetrate into the cells, where a subsequent conjugation reaction takes place. This assumption is plausible since studies have shown the presence of UDP-glucuronosyltransferase(Reference Nowell, Leakey, Warren, Lang and Frame52) and phenolsulphotransferase(Reference Littlewood, Glover, Sandler, Petty, Peatfield and Rose53) in platelets and an in vitro experiment demonstrates that metabolites of radiolabelled-hydroxytyrosol are rapidly formed after the incubation of hydroxytyrosol in whole human blood(Reference D'Angelo, Manna, Migliardi, Mazzoni, Morrica, Capasso, Pontoni, Galletti and Zappia54).
Data presented here indicate that drinking 200 ml coffee (one cup) inhibits platelet aggregation in man. We also demonstrated that coffee phenolic acids penetrate into platelets, and that they are present in platelets at concentrations that are able to inhibit platelet aggregation in vitro. Clearly, we cannot exclude that other non-phenolic coffee compounds, different from caffeine, could contribute to the antiplatelet effect of coffee observed ex vivo.
For a long time the relationship between coffee and cardiovascular risk has been controversial. However, recent epidemiological studies strongly suggest the existence of a J-shaped relationship linking coffee consumption and CVD risk(Reference Kleemola, Jousilahti, Pietinen, Vartiainen and Tuomilehto1–Reference Andersen, Jacobs, Carlsen and Blomhoff3). It is reasonable to hypothesize that such J-shaped correlation is the direct result of the combined positive and negative action of different molecules present in coffee. As it has been demonstrated that ex vivo platelet aggregation is related to CVD mortality(Reference Thaulow, Erikssen, Sandvik, Stormorken and Cohn10), the anti-aggregative action of coffee phenolic acids could represent the bright side of coffee.
Acknowledgements
The study was supported by grants from the Institute for Scientific Information on Coffee (ISIC) in La Tour-de-Peilz (Switzerland) and the Physiological Effects of Coffee Committee (PEC), in La Tour-de-Peilz (Switzerland). There is not any actual or potential conflicts of interest capable of influencing judgement on the part of any author. We thank all the volunteers for their participation. Daniela Salzano and Monica Brancorsini are thanked for their nursing assistance. Kariklia Pascucci is acknowledged for her kind support in the daily laboratory work. We would like also to thank Sigma-Tau (Pomezia, Italy) for donating caffeine capsules.









